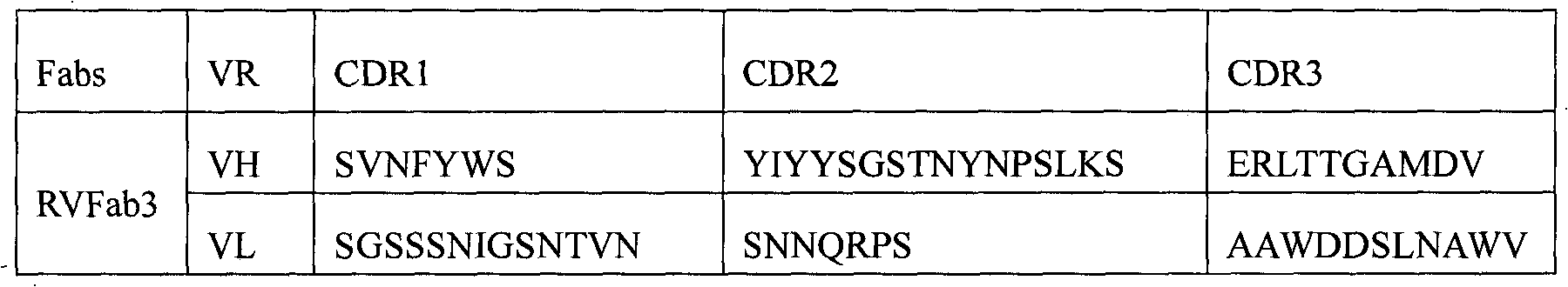
Humanized neutralizing antibody (RVFab3) against rabies virus glycoprotein
A technology of rabies virus and glycoprotein, applied in the direction of antiviral immunoglobulin, antiviral agent, antibody, etc., can solve the problems of infection, limited application, and large-scale production restrictions, and achieve the effect of strong penetration
Active Publication Date: 2010-08-25
STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
View PDF1 Cites 3 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
However, most McAbs are of mouse origin, and the heterogeneous reaction of mouse McAbs greatly limits the application of McAbs as therapeutic agents in humans.
As an antibody component, immunoglobulin (Vaccinia immune globulin, VIG) mainly comes from the immune serum of donors (convalescent patients), and it takes a lot of manpower and financial resources to obtain positive serum and pass the safety test, which makes its mass production difficult. Restricted, and because it comes from serum, it is prone to infection of blood-borne diseases
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment Construction
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
 Login to View More
Login to View More Abstract
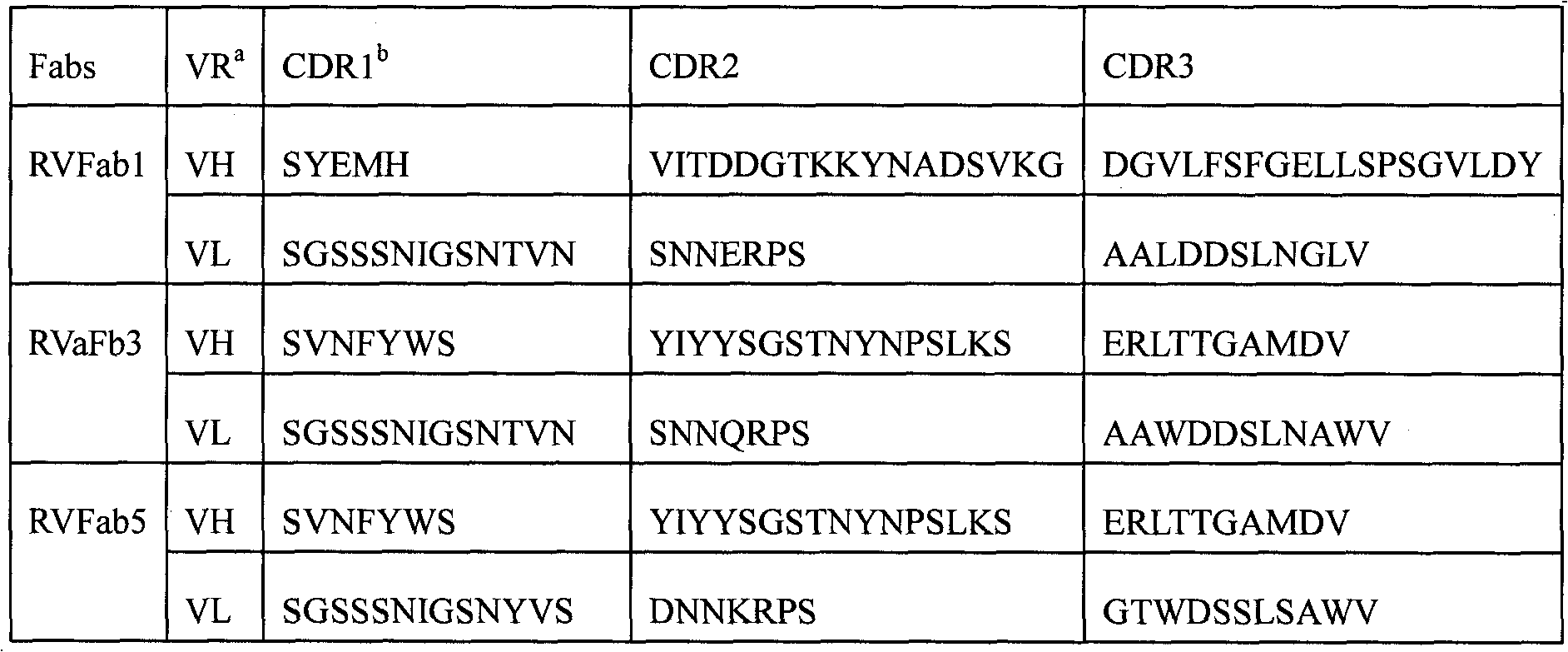
The invention discloses a humanized neutralizing antibody (RVFab3) against rabies virus glycoprotein, which is obtained through screening by utilizing phage display technology. The antibody specifically identifies the granule antigen of the rabies virus, is against the rabies virus glycoprotein G, has obvious immunofluorescence reaction and enzyme linked immunosorbent assay with the rabies virus and has the neutralizing activity function against rabies virus infection. The antibody can be prepared into the specific antibody drugs for preventing and treating rabies, thereby being clinically used for preventing and treating rabies caused by the rabies virus.
Description
technical field The invention relates to genetic engineering antibody technology, in particular to a human-source neutralizing antibody against rabies virus glycoprotein; the invention also relates to the application of the antibody in the preparation of medicaments for preventing or treating rabies. Background technique Rabies is a worldwide zoonotic disease caused by rabies virus, once the onset of 100% death. Rabies has been reported in 87 countries in the world, and more than 50,000 people die from rabies every year (Knobel DL, et al.2005). Rabies post-exposure prophylaxis is the main measure to prevent and control rabies. For severely exposed people, the World Health Organization (WHO) recommends the use of rabies vaccine injection combined with anti-rabies immune globulin (rabies immune globulin, RIG). The two types of RIG currently in use are human rabies immune globulin (HRIG) and equine rabies immune globulin (ERIG). ERIG has severe side effects and inhibits the ...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More Patent Type & Authority Applications(China)
IPC IPC(8): C07K16/10C12N15/13C12N15/63C12N1/15C12N1/19C12N1/21C12N5/10G01N33/569A61K39/42A61P31/14
CPCC07K16/10C07K2317/24A61P31/14
Inventor 梁米芳孙丽娜陈哲李川李德新
Owner STATION OF VIRUS PREVENTION & CONTROL CHINA DISEASES PREVENTION & CONTROL CENT
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com