Avian influenza virus-like particle vaccine as well as preparation method and application thereof
An avian influenza virus and particle technology, applied in the fields of botanical equipment and methods, biochemical equipment and methods, viruses, etc., can solve problems such as failure to achieve prevention and control effects, high production costs, and long production cycles, and achieve no biological safety. Risk, low production cost, good immunogenicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Example 1 Expression of H5 subtype avian influenza virus-like particles
[0046] 1. Carrier modification
[0047] Using the commercialized vector pFastBac I as a template, NdeI, NotI, SalI and XbaI restriction enzyme sites were sequentially inserted at positions 4413-4414. Add 1 μl of DpnI enzyme to the PCR product to digest the template plasmid, take 5 μl and transform it into DH 5α according to the conventional method, and the successfully transformed plasmid is named pFastBac mut.
[0048] 2. Construction of three expression cassette donor plasmids
[0049] The HA gene shown in Seq ID NO.1, the NA gene shown in Seq ID NO.2, and the M1 gene shown in Seq ID NO.3 were synthesized by Suzhou Jinweizhi Biotechnology Co., Ltd., and the upstream and downstream of the genes were respectively added Dicer BamHI and HindIII restriction sites. The synthesized HA gene, NA gene, and M1 gene were respectively digested with BamHI and HindIII, and then ligated with the pFastBac mut...
Embodiment 2
[0059] Example 2 Preparation of H5 subtype avian influenza virus-like particle vaccine
[0060] The virus-like particles harvested in Example 1 were respectively added to the mineral oil adjuvant to prepare various vaccine compositions, and the specific proportions are shown in Table 1.
[0061] Table 1 Proportion of H5 subtype avian influenza virus-like particle vaccine composition
[0062] components Vaccine 1 Vaccine 2 Vaccine 3 Vaccine 4 Vaccine 5 Vaccine 6 rBac-M1-1 (HA content) 6log2 8log2 - - 6log2 8log2 rBac-M1-2 (HA content) - - 6log2 8log2 6log2 8log2 Adjuvant (V / V%) 66% 66% 66% 66% 66% 66%
Embodiment 3
[0063] Example 3 H5 subtype avian influenza virus-like particle vaccine immunogenicity test
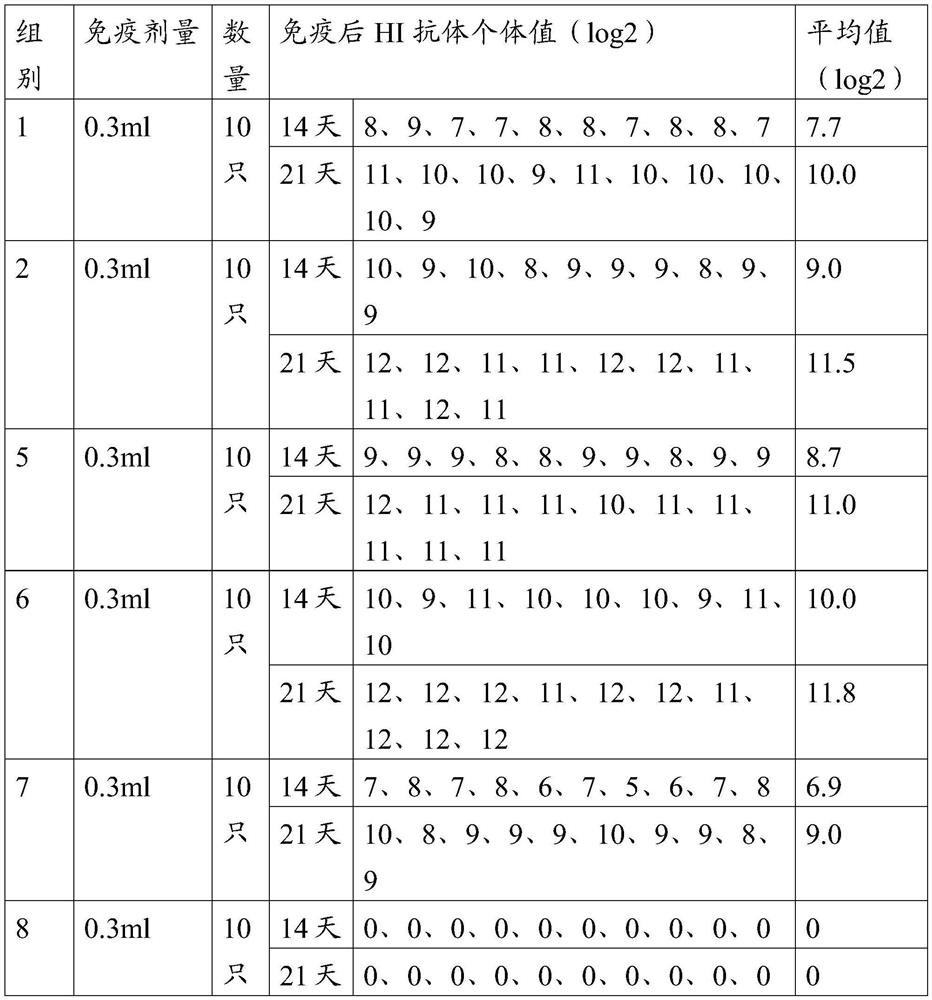
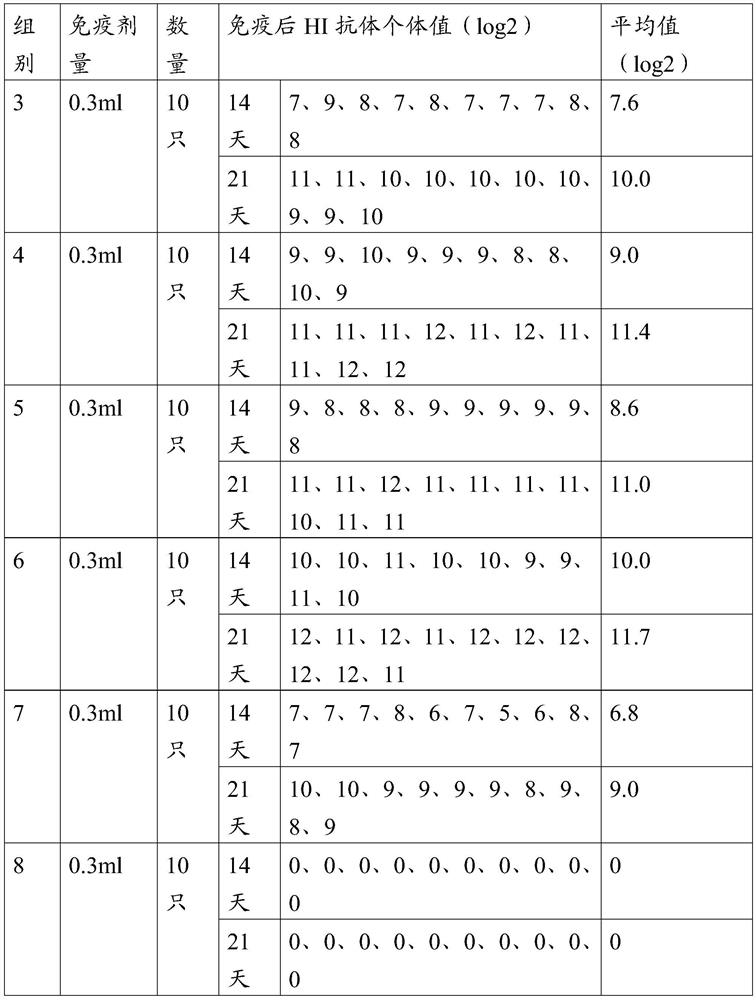
[0064] Get 80 SPF chickens at the age of 21 days, divide them into 8 groups, 10 in each group, the 1st group to the 6th group are respectively injected vaccine 1~vaccine 6 prepared in Example 2 through the neck subcutaneously, and the 7th group subcutaneously injects commercial products Chemical inactivated vaccine (H5 (Re-11+Re-12), H7 (H7-Re2) trivalent inactivated vaccine, H5HA content is 8log2), immunization dose is 0.3ml, the 8th group is subcutaneously injected with 0.3ml normal saline , as a blank control. All experimental chickens were kept in isolation, blood was collected on the 14th and 21st day after immunization, serum was separated, and the titer of HI antibody was determined*. The test results of different HI antibodies after immunization are shown in Tables 2 and 3.
[0065] Table 2 H5 subtype avian influenza virus-like particle vaccine immunogenicity test results 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com