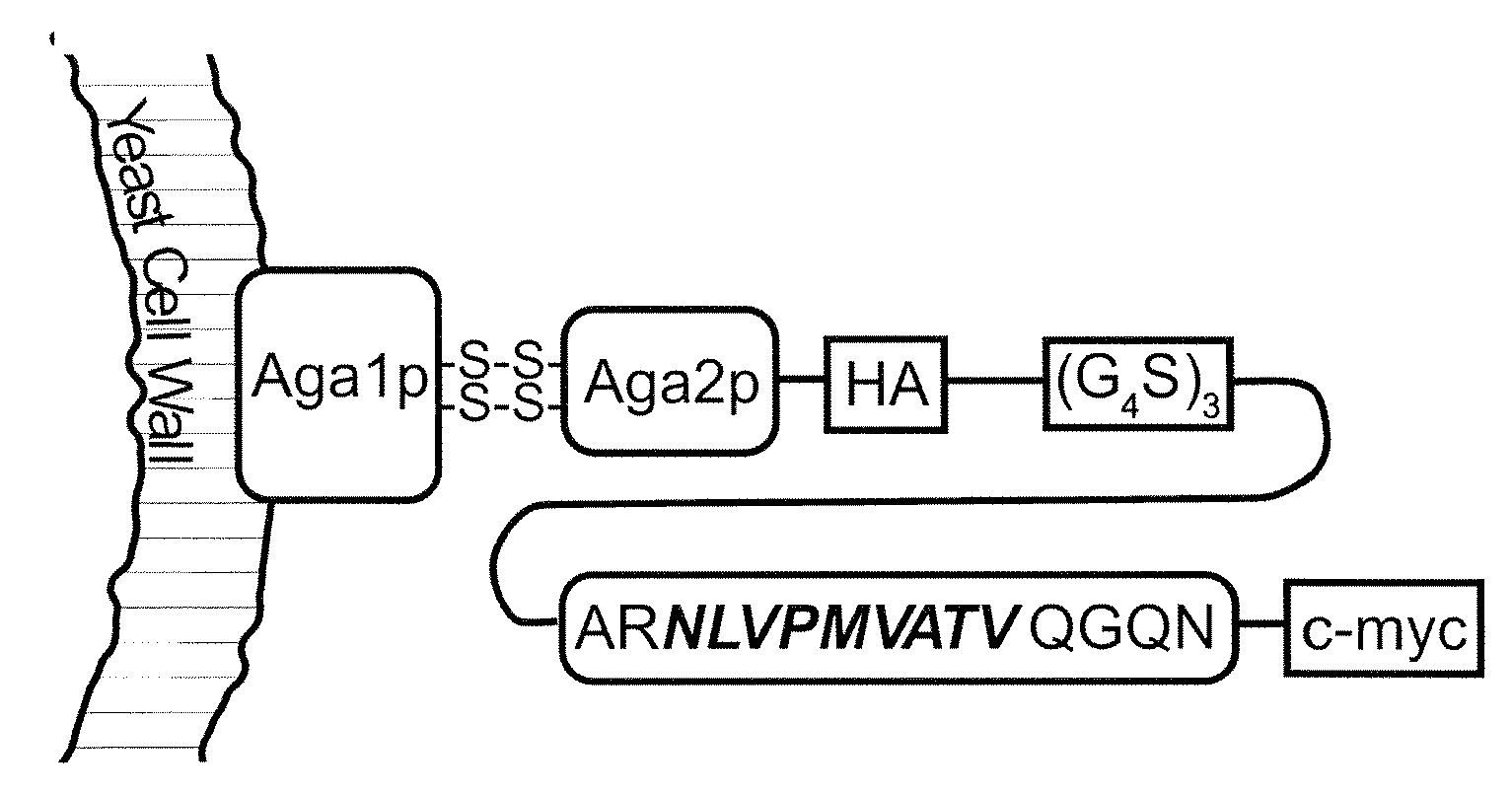
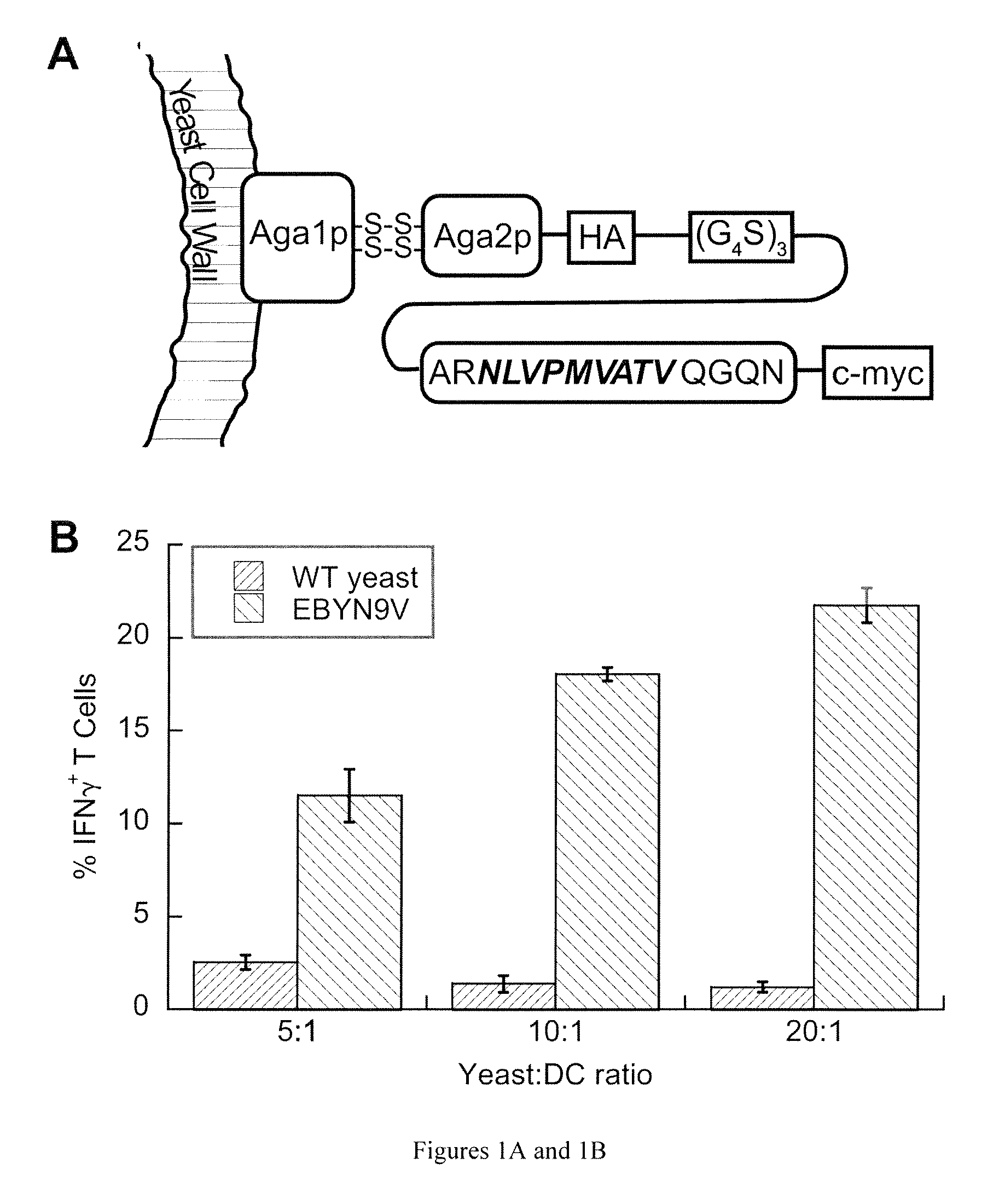
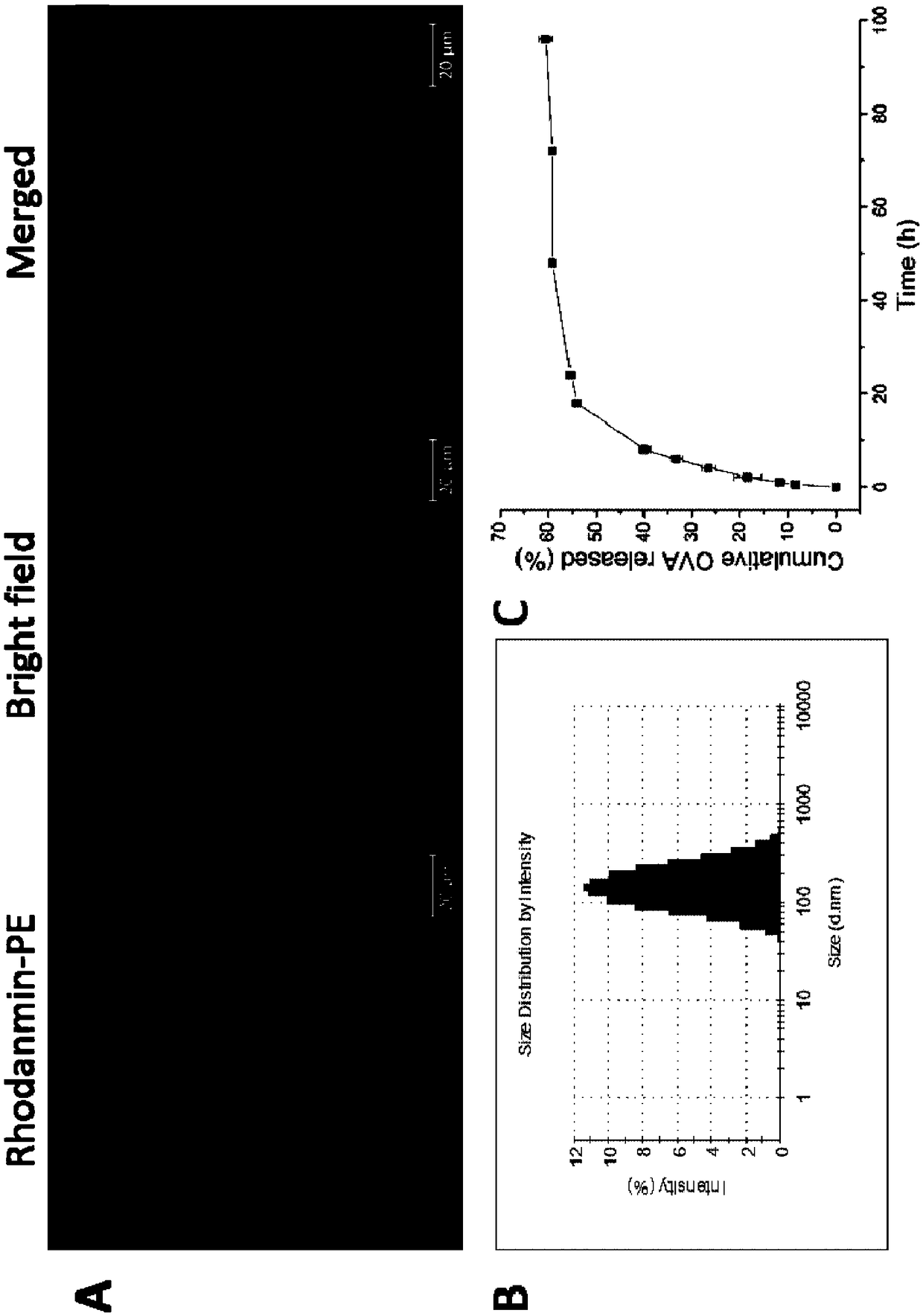
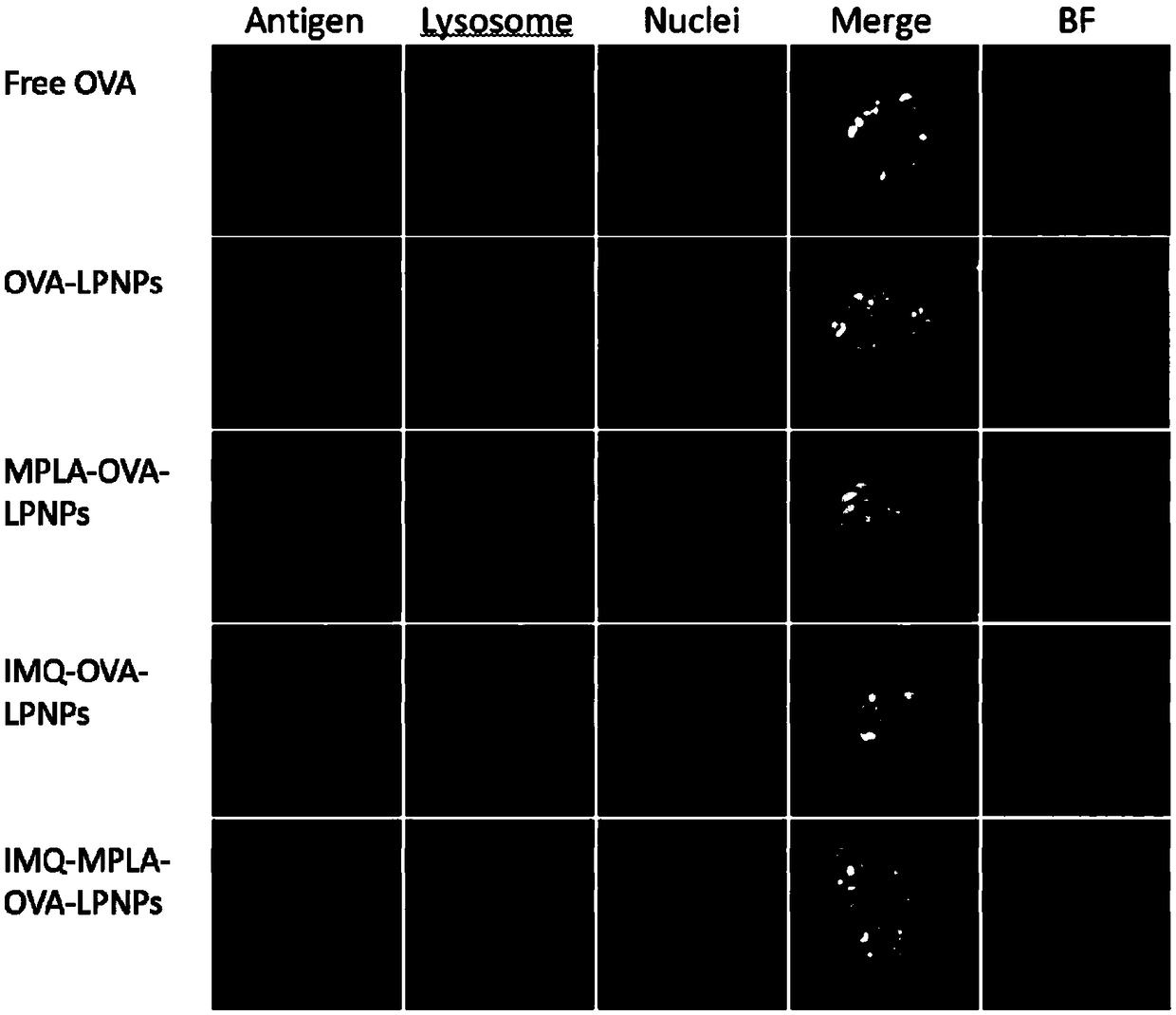
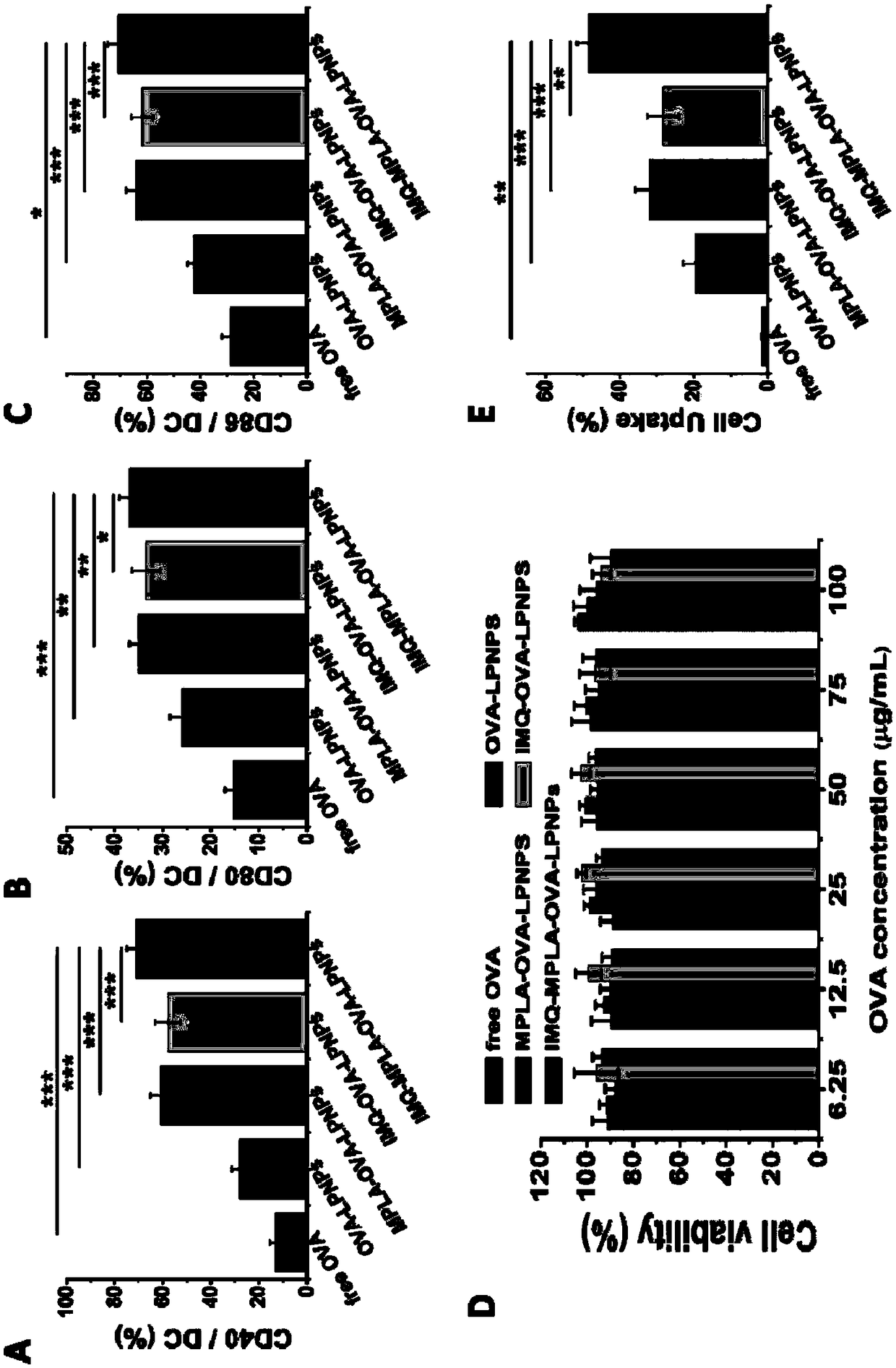
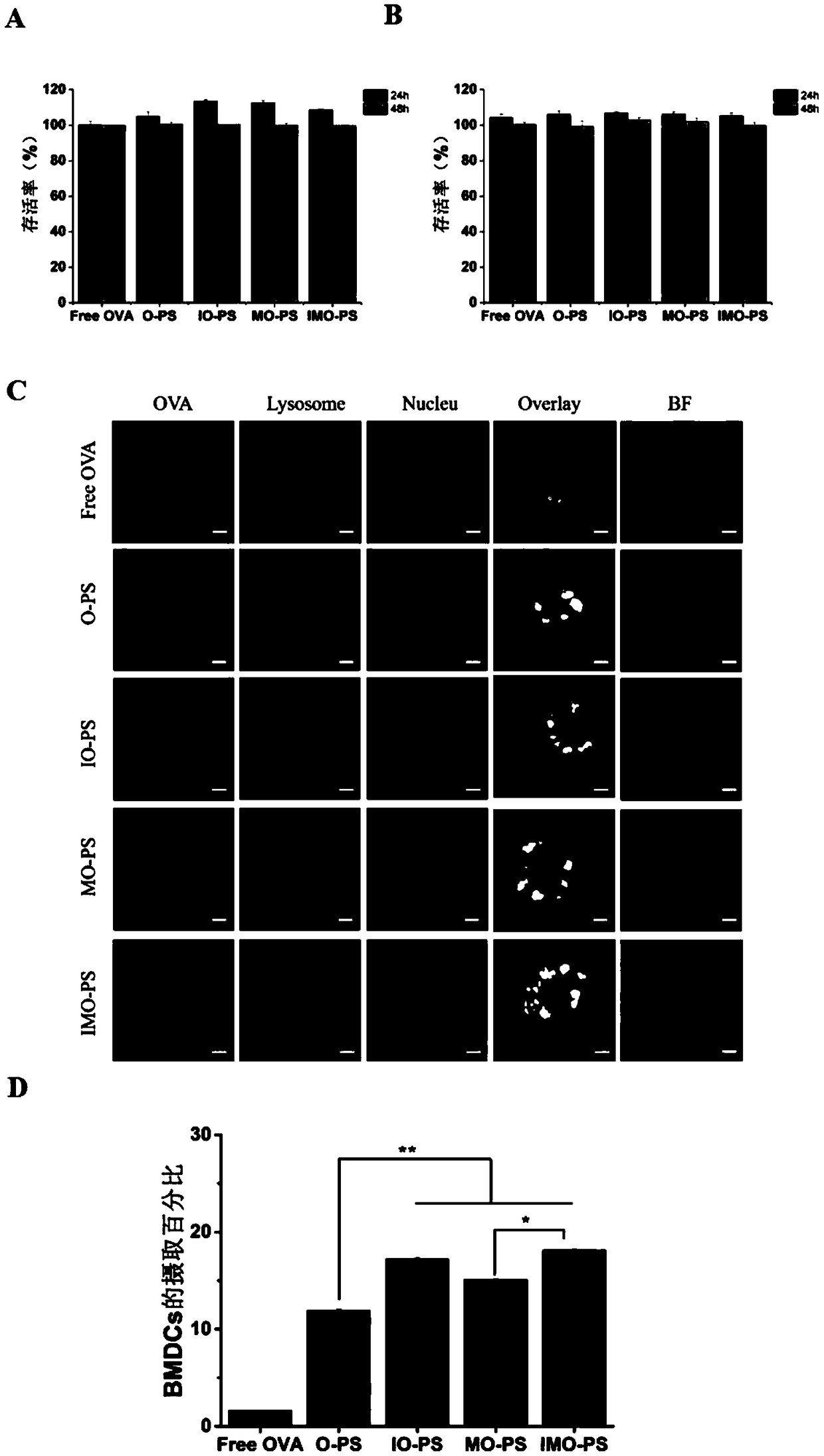
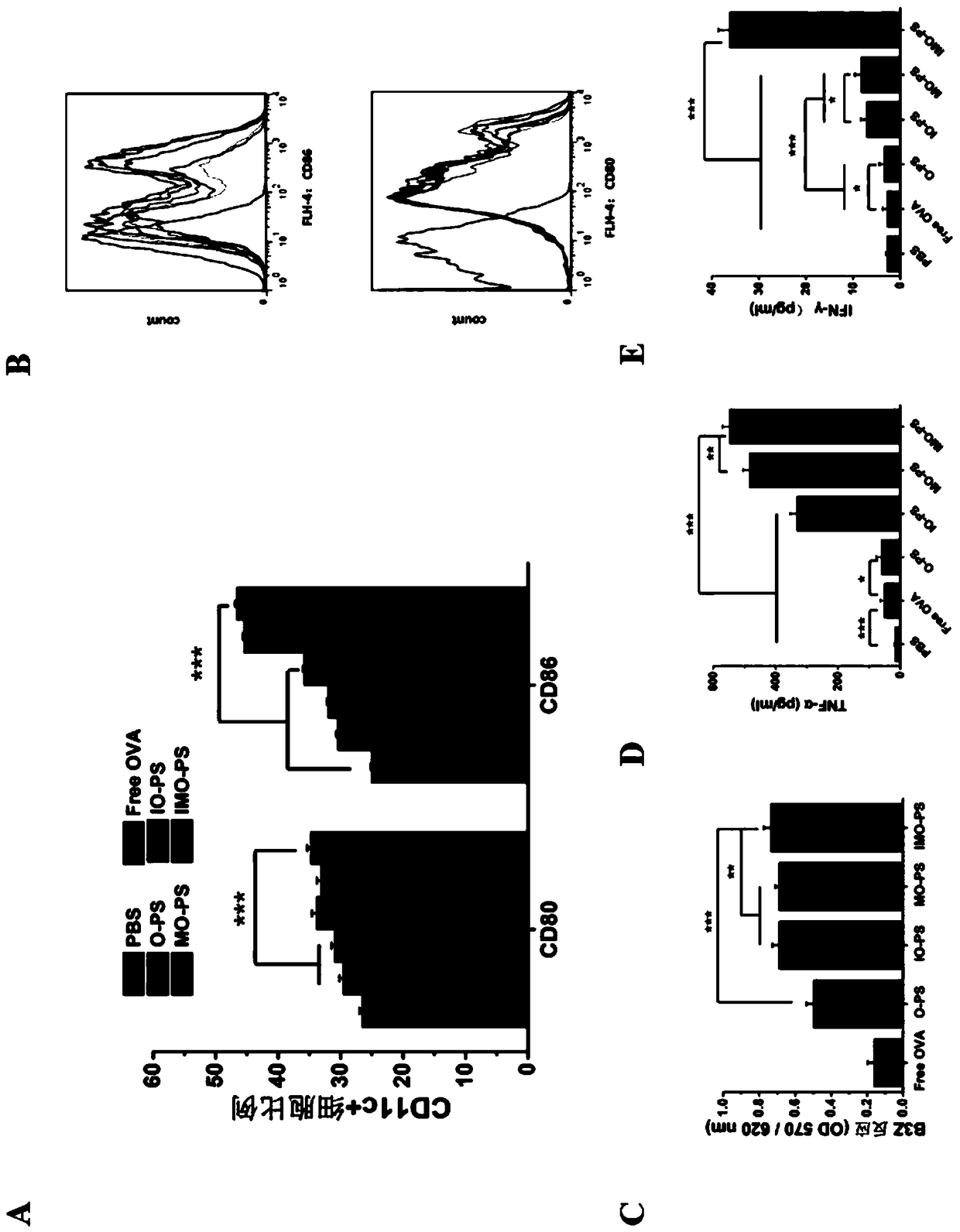
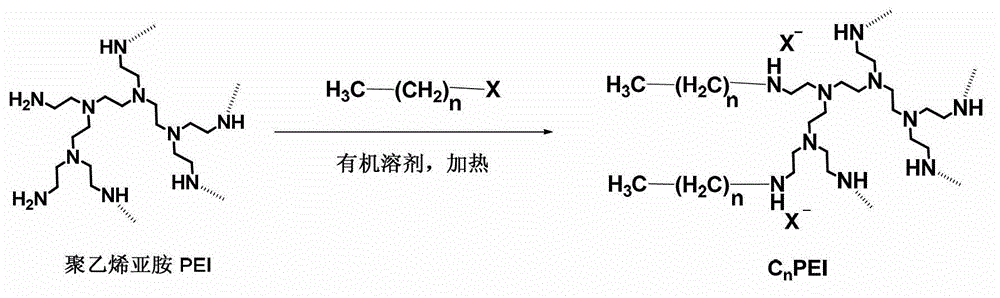
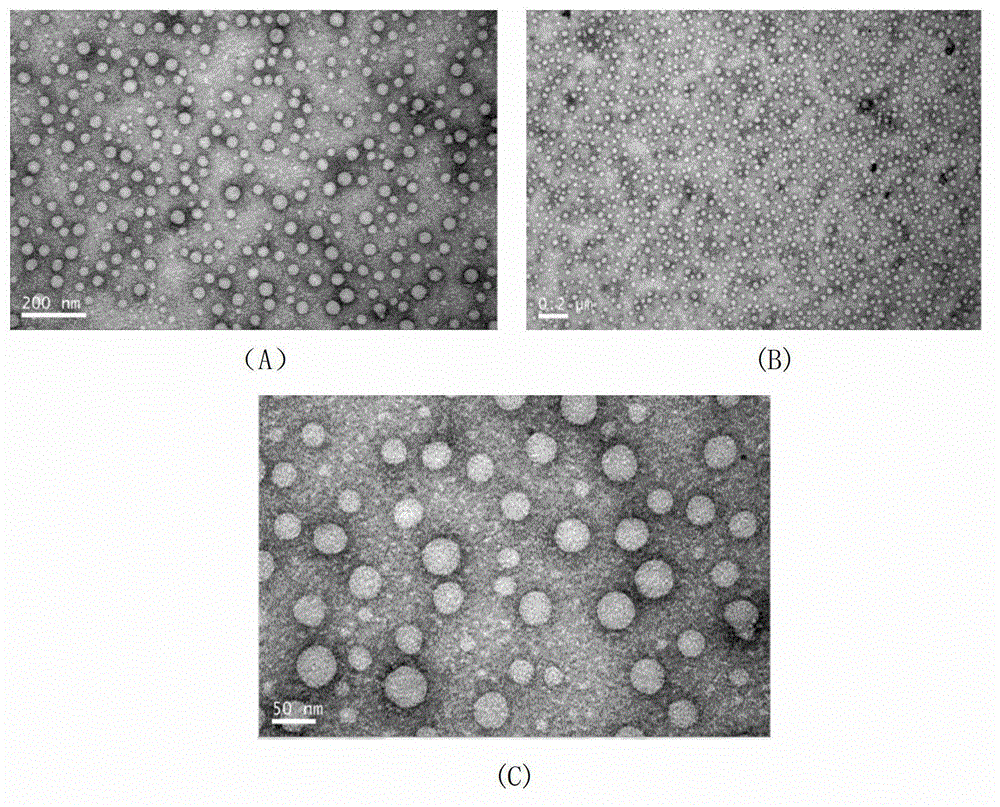
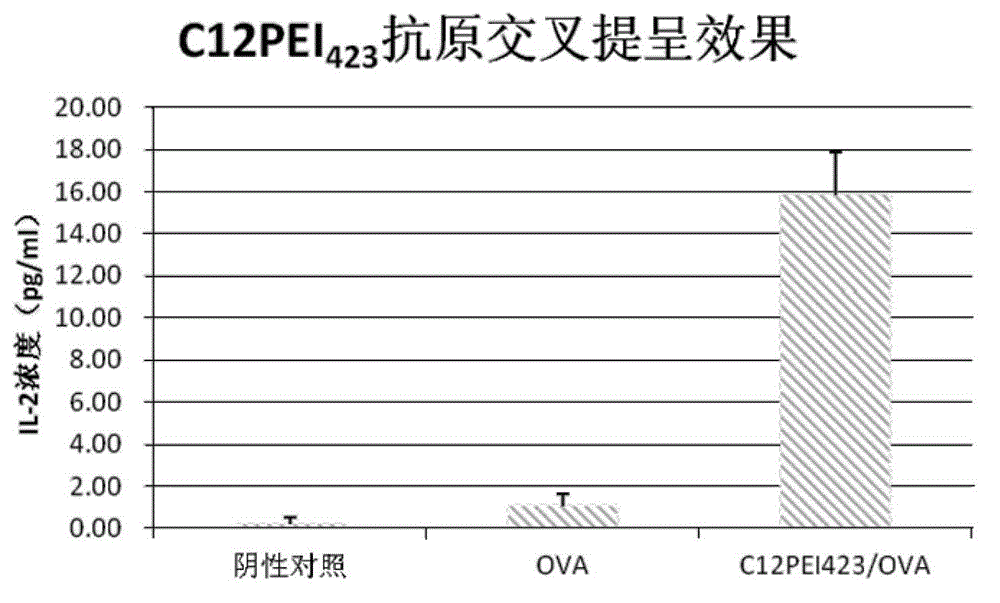
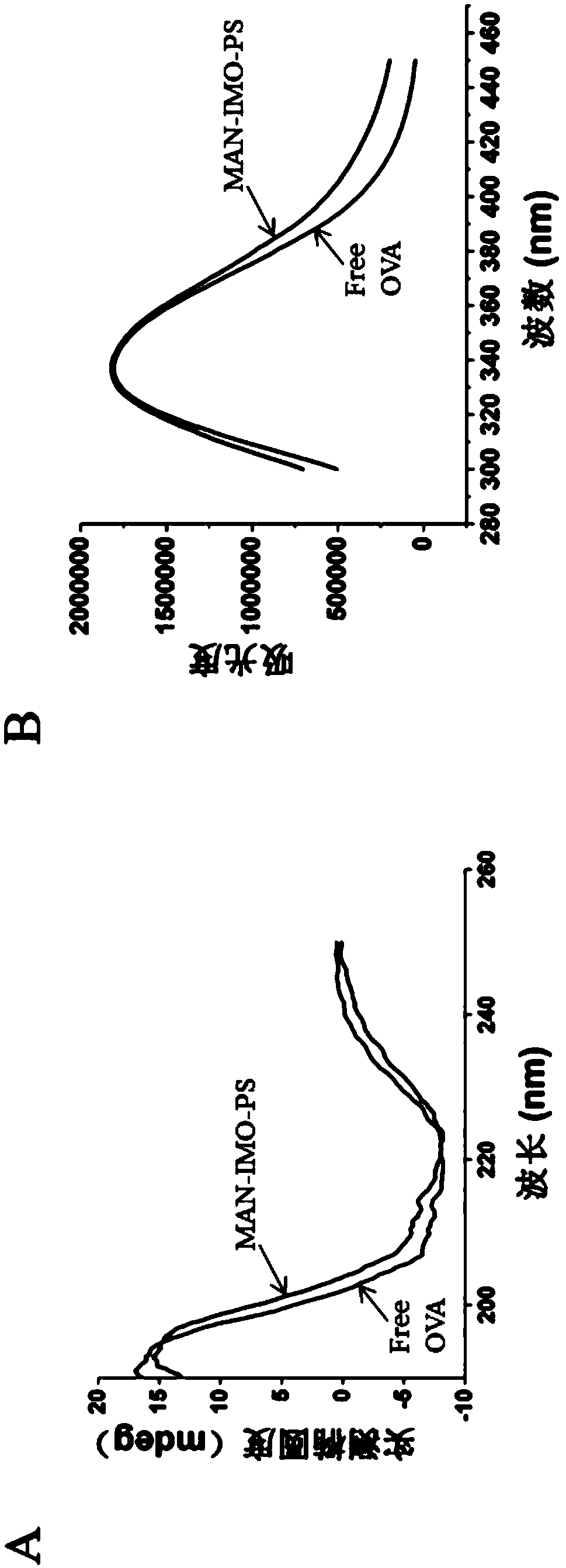
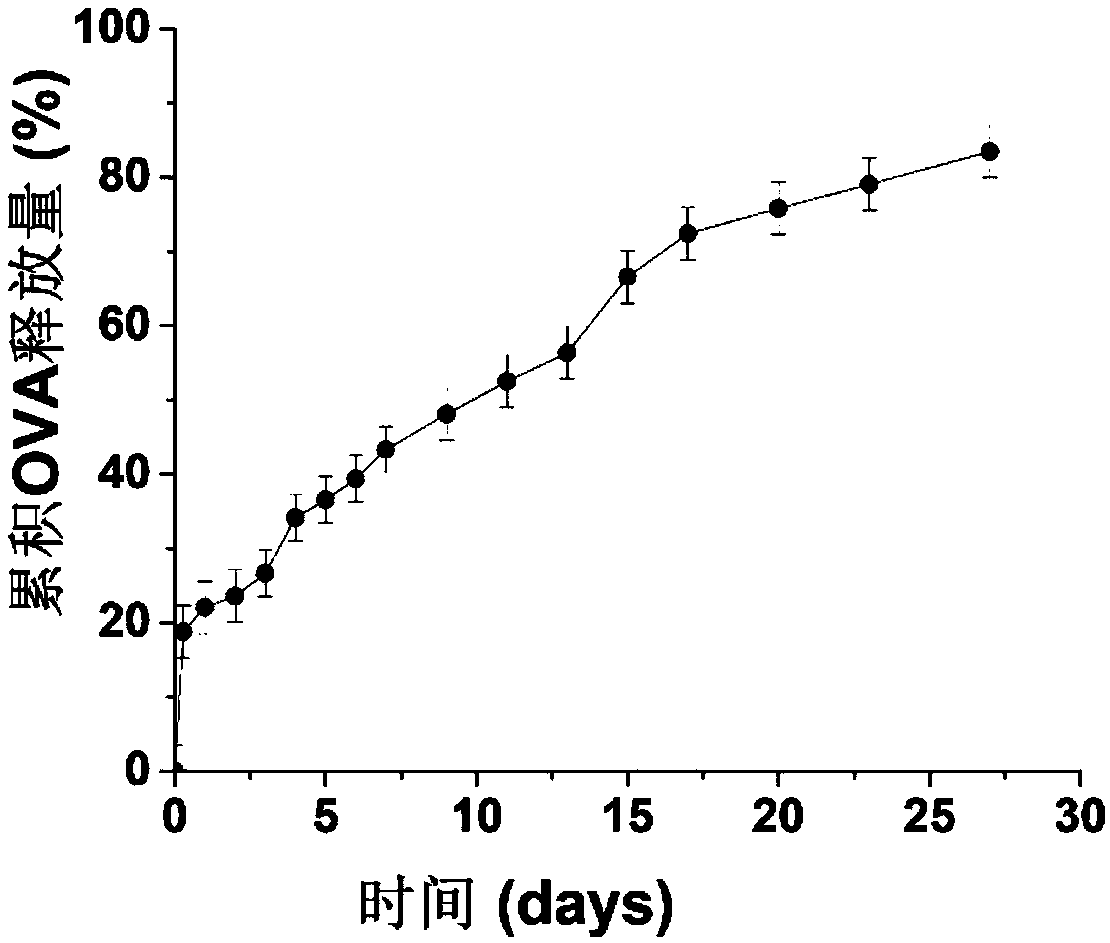
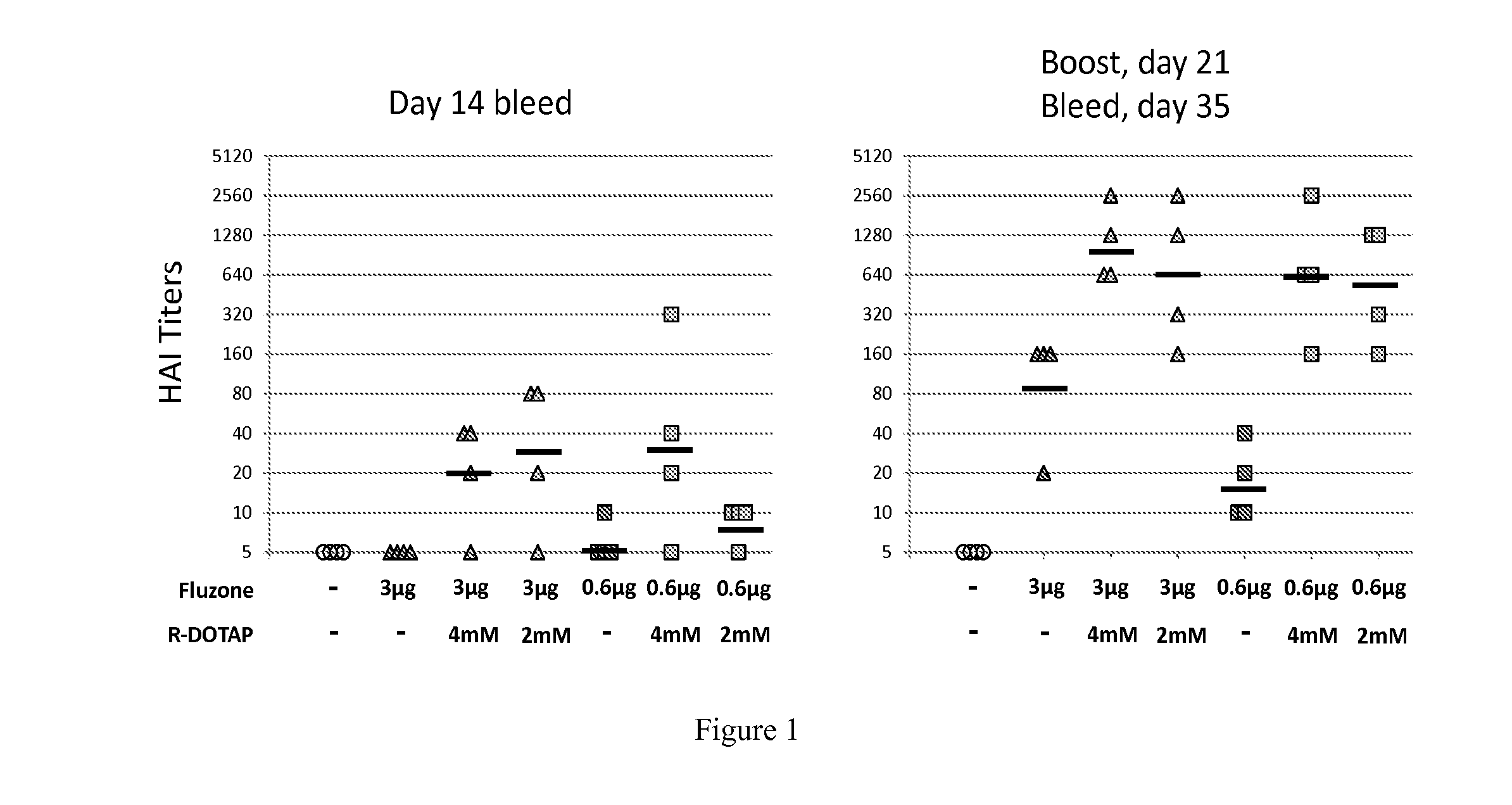
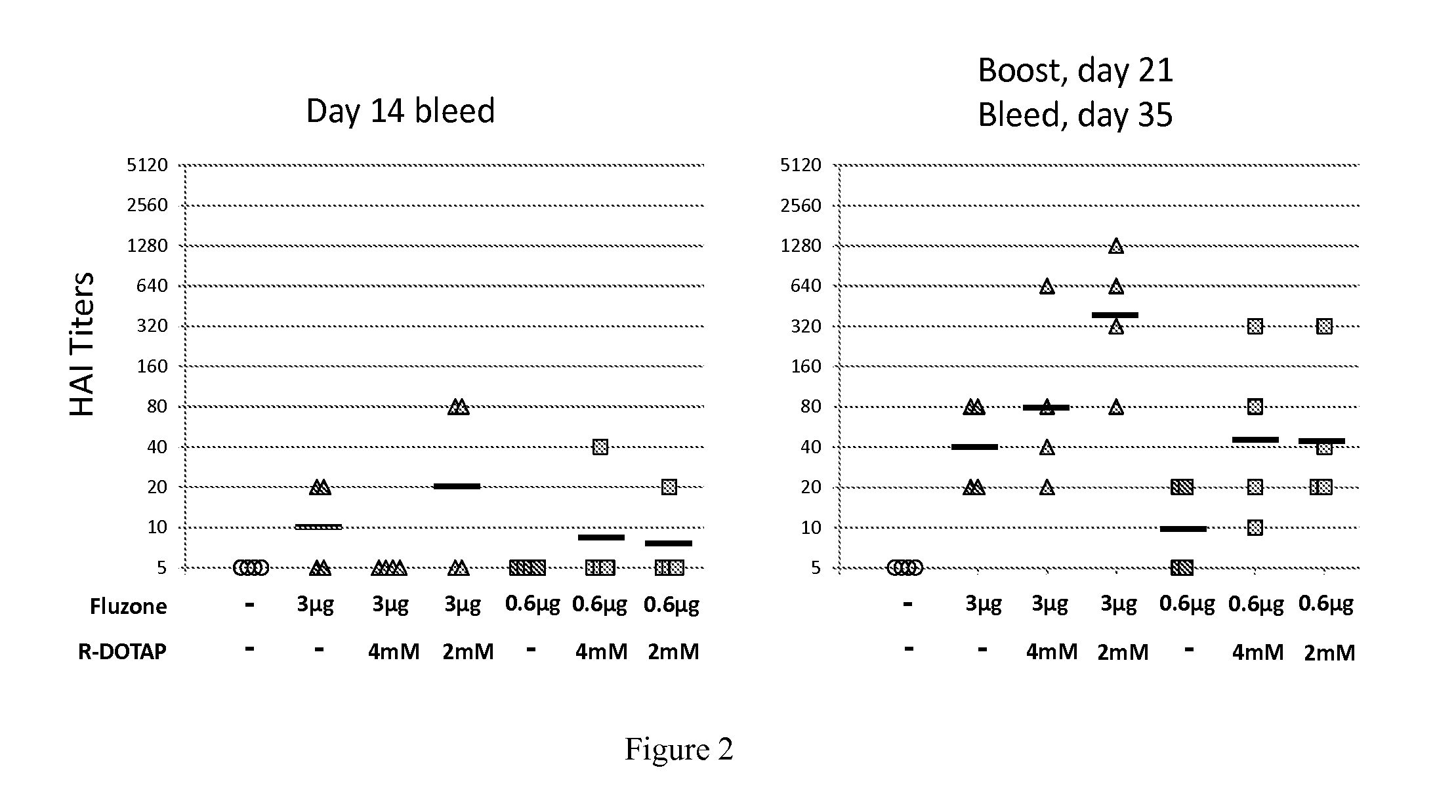
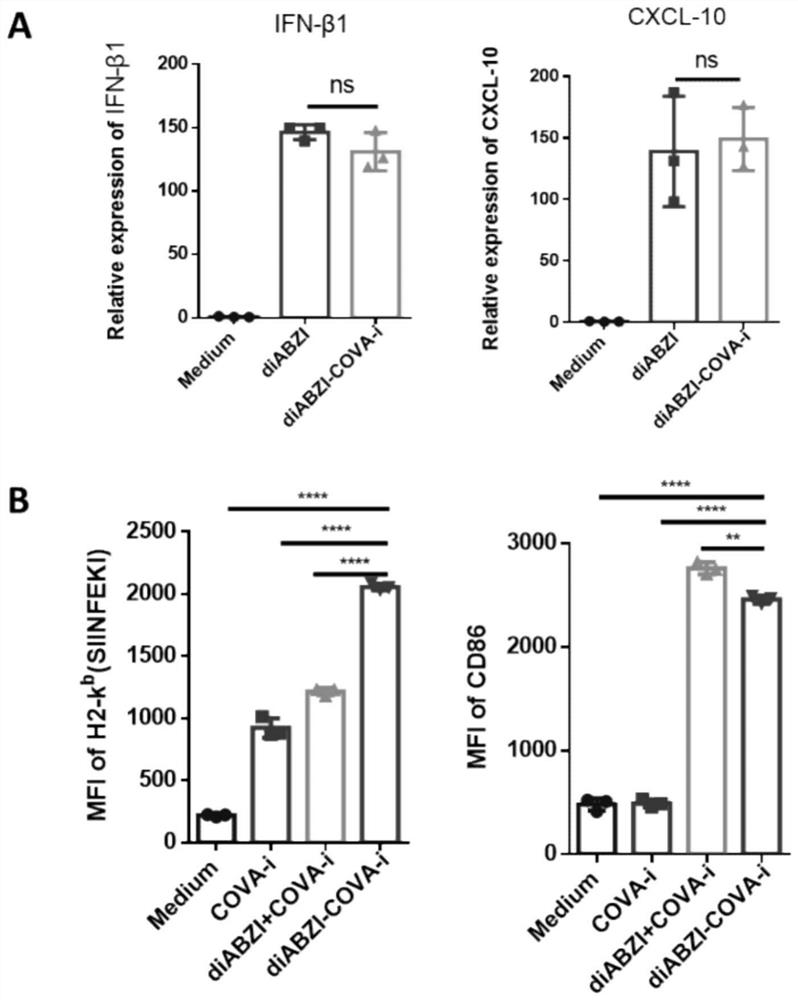
Patents
Literature
46 results about "Cross-presentation" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
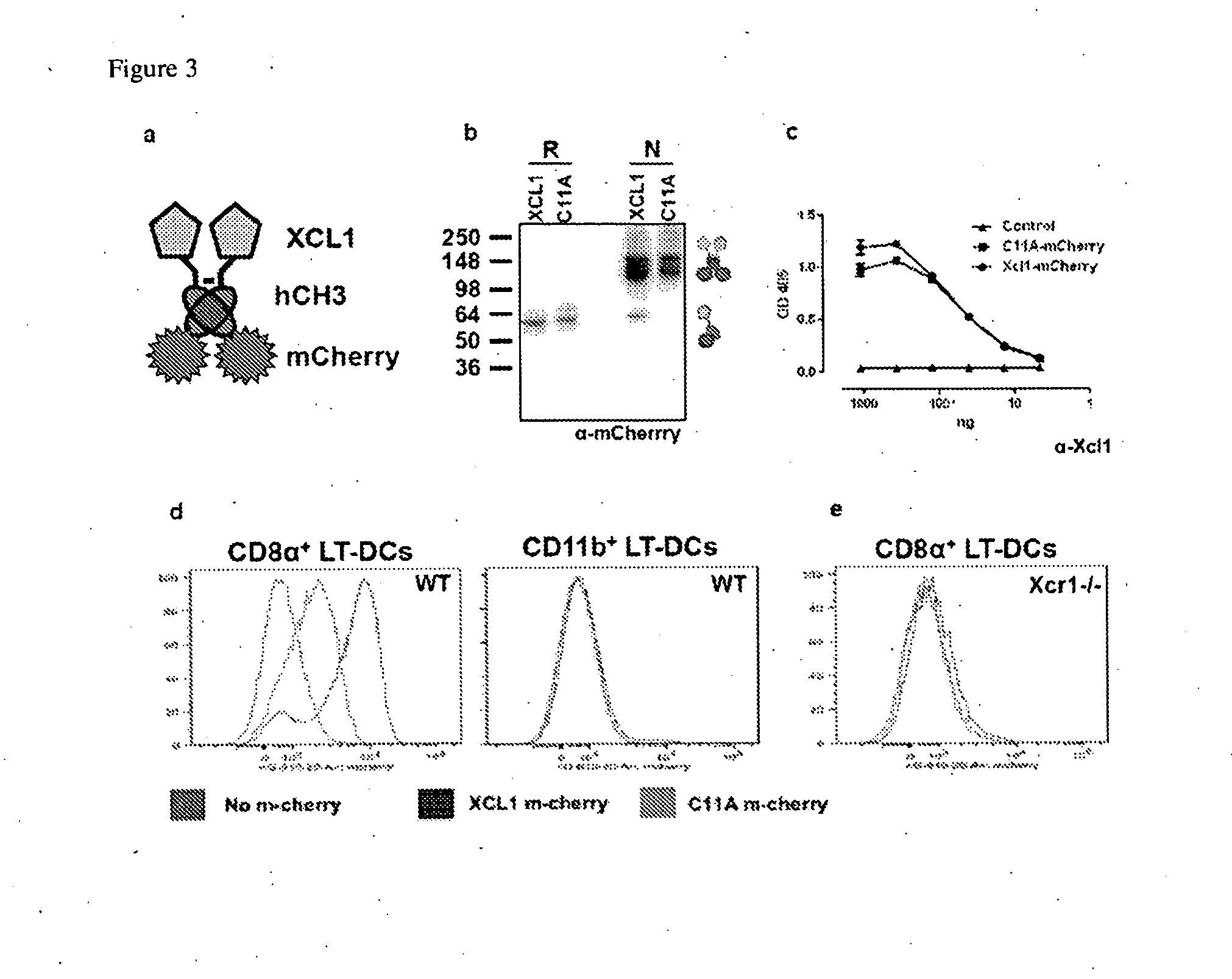
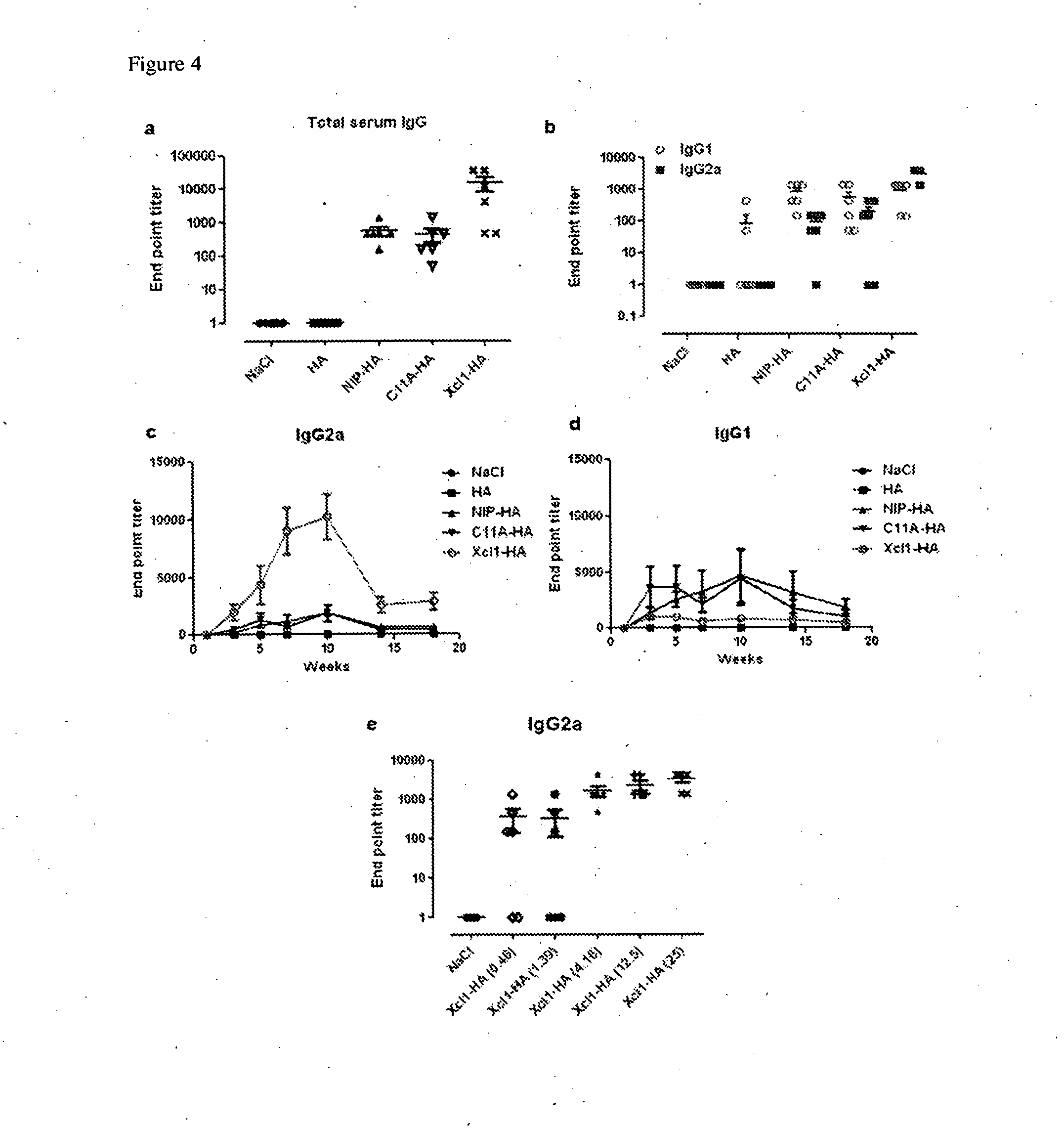
Cross-presentation is the ability of certain antigen-presenting cells to take up, process and present extracellular antigens with MHC class I molecules to CD8 T cells (cytotoxic T cells). Cross-priming, the result of this process, describes the stimulation of naive cytotoxic CD8⁺ T cells into activated cytotoxic CD8⁺ T cells. This process is necessary for immunity against most tumors and viruses that do not readily infect antigen-presenting cells, but rather intracellular tumors and viruses that infect peripheral tissue cells. Cross presentation is also required for the induction of cytotoxic immunity by vaccination with protein antigens, for example, tumour vaccination.
Methods and compositions for increased priming of t-cells through cross-presentation of exogenous antigens
InactiveUS20080171059A1Easy to demonstrateEffective vaccineTissue cultureCancer antigen ingredientsDiseaseVaccination
Methods for eliciting in an animal in need thereof a cell-mediated immune response specific to an antigen, the method comprising providing an antigen preparation comprising particles on the surface of which the antigen is attached, and administering the antigen preparation to the animal, wherein the particles are taken up by antigen presenting cells (APC) of the animal via phagocytosis, forming a phagosome inside the APC, wherein the antigen is attached to the surface of the particle in such a way that the antigen is released in the phagosome before the phagosome fuses with a late endosome or a lysosome, and wherein the antigen is cross-presented on a Class I MHC molecule. Also provided are particulate antigen preparations or particulate vaccines that can be delivered to an animal in need thereof for vaccination against, for preventing or treating, a disease related to the antigen, such as cancer and a viral infection.
Owner:LUDWIG INST FOR CANCER RES +1
Mosaic type virus-like particle DNA vaccine
InactiveCN1903363AIncreased ability to elicit a complete immune responseResist infectionGenetic material ingredientsAntiviralsHepatitis B virus core AntigenT cell
A chimeric virus-like granular DNA vaccine able to generate stronger B cell activity, exciting high antibody level, improving cell's immunizing level by cross presentation, and activating T cell, toxic T cell and complete immunoreaction is a recombinant eucaryotic expression carrier of coding gene with chimeric protein. Its preparing process is also disclosed.
Owner:CHINA AGRI UNIV
Vaccine vector based on aluminum hydoxide nano-particles
ActiveCN104587464AReduce aluminum contentNo inflammationPharmaceutical non-active ingredientsImmunological disordersDendritic cellTGE VACCINE
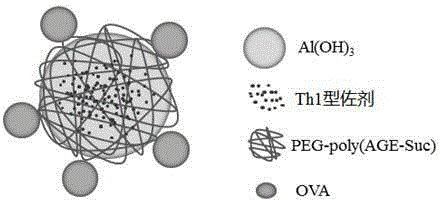
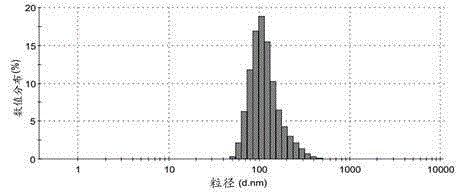
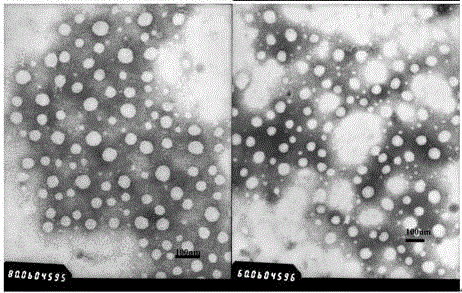
The invention provides an aluminum adjuvant used as a vaccine vector. The adjuvant is characterized in that a PEG derivative bio-material and aluminum are compounded to form nano-particles, so that the property of strong Th2 body fluid immunologic adjuvant of aluminum salt is maintained, and the adjuvant can be efficiently transferred to draining lymph nodes in the body and can be easily ingested by dendritic cells to perform effective cross-presentation and induce cellular immunologic response. The aluminum adjuvant has strong Th1 immunologic response.
Owner:SICHUAN UNIV
Cationic phospholipid-polymer hybridized nanoparticle vaccine adjuvant of common-carrier antigen, MPLA (Monophosphoryl Lipid A) and IMQ (Imiquimod) as well as preparation method and application thereof
ActiveCN108743939AGood biocompatibilityPromote degradationPharmaceutical non-active ingredientsAntibody medical ingredientsDendritic cellPhospholipid
The invention relates to a cationic phospholipid-polymer hybridized nanoparticle vaccine adjuvant of a common-carrier antigen, MPLA (Monophosphoryl Lipid A) and IMQ (Imiquimod) as well as a preparation method and application thereof. The vaccine adjuvant is characterized in that the IMQ as a TLR7 agonist is loaded on a hydrophobic core; the MPLA as a TLR4 agonist is loaded in a phopholipid layer;cationic phospholipid DOTAP (1,2-dioleoy-3-trimethylammonium-propane) in the phopholipid layer is used for adsorbing an antigen; the antigen is protected through hybridized nanoparticles, and the ingestion of the antigen by dendritic cells is improved; immune response after antigen stimulation is improved remarkably through the TLR agonist, and cross-presentation of the antigen is improved remarkably. The hybridized nanoparticles as the vaccine adjuvant can load the antigen and different types of TLR agonists simultaneously, can deliver the antigen through a plurality of immune paths, and promotes the DC activation and maturation. The cross-presentation level is raised, a strong and powerful T-cell killing effect is achieved, cell factor secretion is induced, a long-term memory T-cell reaction is generated, and higher prevention capability for tumors is achieved.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
DCs vaccine based on phospholipid hybrid polymersome jointly encapsulating antigen and dual immunoagonists and preparation method and application thereof
ActiveCN108938568AMaximize Targeting EffectAchieving ImmunotherapyCancer antigen ingredientsPharmaceutical non-active ingredientsT lymphocyteBiological activation
The invention relates to a DCs vaccine based on phospholipid hybrid polymersome jointly encapsulating an antigen and dual immunoagonists, a preparation method and application thereof. The phospholipidhybrid polymersome which can jointly load a model antigen OVA and two types of TLR agonists (TLR7 / 8 and TLR4) is used for stimulation in vitro of the DCs so as to realize the effective phagocytosis of DCs cells. The rapid and long-term immunostimulatory effect on the DCs is achieved by the internal and external co-loading of the OVA antigen. The synergistic effect of the two types of TLR agonistssignificantly enhances the immune response after antigen stimulation; the phospholipid hybrid polymersome which jointly encapsulates the antigen and the dual immunoagonists can effectively promote the activation and maturation of the DCs, increases the level of cross-presentation, promotes the migration of the DC vaccine to secondary lymphoid organs, and produces a strong specific cytotoxic T lymphocytes (CTLs) killing effect, thereby effectively killing tumor cells and realizing the immunotherapy of the DCs vaccine on tumors.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Hydrophobic-modified polyethyleneimine and application thereof as protein carrier
InactiveCN103059295AStrong protein bindingIntroduce an immune responseViral antigen ingredientsAntiviralsAntigenNanocarriers
The invention discloses a hydrophobic-modified polyethyleneimine and application thereof as a protein carrier. The structural general formula of the hydrophobic-modified polyethyleneimine is disclosed in the specification, wherein n is any natural number ranging from 5 to 20, and X is Cl, Br or I. The hydrophobic-modified polyethyleneimine can be compounded with protein to form stable nanoparticles, and thus, can be used as a protein carrier. Compared with the prior art, the hydrophobic-modified polyethyleneimine disclosed by the invention has strong protein binding force, can carry antigen protein, can be recognized by antigen presenting cells, and can initiate immunoreaction; and meanwhile, the hydrophobic-modified polyethyleneimine enhances the antigen cross-presentation effect, obviously lowers the cytotoxicity, is beneficial to enhancing the immunotherapeutic effect, and is an excellent therapeutic tumor vaccine nano carrier system.
Owner:SHANGHAI JIAO TONG UNIV +1
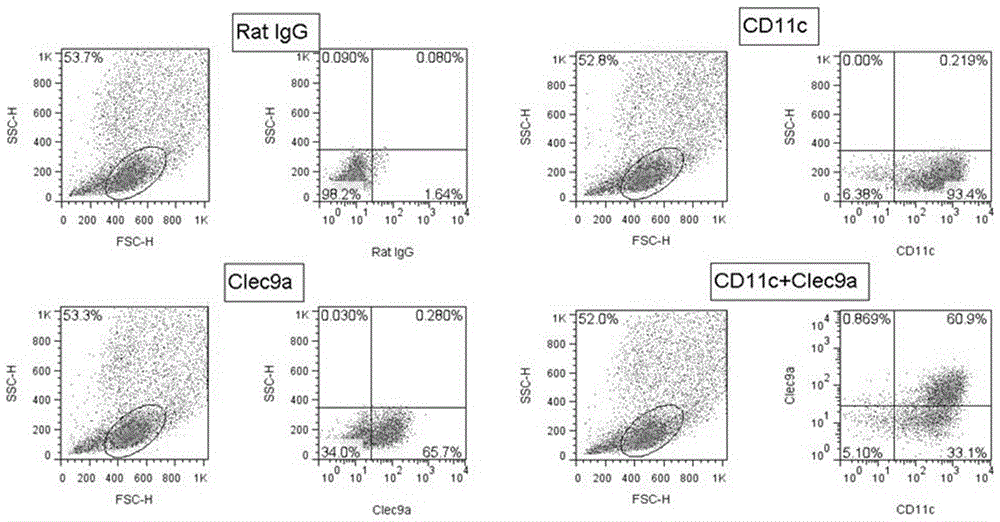
Immune modulation via C-type lectin
The invention relates to the regulation of the immune system, and in particular to the finding that the CLEC9a molecule is a marker for dendritic cells which are capable of cross-presenting extracellular antigens via the MHC class I pathway. This makes them particularly suitable for generation of cytotoxic T lymphocyte responses. Materials and methods are provided both for the induction of immune responses against target antigens, and for the inhibition or suppression of undesirable immune responses in which these cells are involved.
Owner:CANCER RES TECH LTD
Methods For Producing Dendritic Cells That Have Acquired Ctl-Inducing Ability
InactiveUS20080194020A1Promotion of antigenic protein polyubiquitinationEnhance antigen presentationSugar derivativesPeptide preparation methodsProteasomeCross-presentation
The present inventors worked on elucidating the mechanism of antigen cross-presentation by dendritic cells, and revealed that foreign antigens taken up in dendritic cells are degraded by proteasomes after undergoing polyubiquitination. Based on the novel finding that polyubiquitination is involved in cross-presentation, the promotion of polyubiquitination was attempted by a number of methods, and the promotion of antigen presentation was confirmed. These methods enable the production of dendritic cells effective for inducing CTL activation.
Owner:KEIO UNIV +1
Mannose modified co-loaded antigen and double immuno-agonist phospholipid hybrid polymer vesicle as well as preparation method and application thereof
ActiveCN108969771AMaximize Targeting EffectMaximize the effect of targeted immunotherapyAntibacterial agentsBacterial antigen ingredientsAdjuvantBiocompatibility Testing
The invention relates to a mannose modified co-loaded antigen and a double immuno-agonist phospholipid hybrid polymer vesicle as well as a preparation method and application thereof. A vaccine carriertakes an amphiphilic triblock copolymer as material, OVA is loaded in the hydrophilic inner cavity, IMQ is loaded in the hydrophobic membrane layer, DOTAP cationic layer is fitted with MPLA, and cationic lipid outer layer adsorbs outer layer OVA; phospholipid with reactive group is introduced to pass the targeted mannose ligand through covalently linked to the PEG-active distal end of the polymer-loaded vesicle surface, integrating the functions of active targeting of tumors, co-delivery of antigens and adjuvants and the like; the vesicle has the characteristics of small particle size, good dispersion, high antigen loading and good biocompatibility, etc., which can promote antigen uptake, DC activation and maturation, antigen cross-presentation, antigenic lymph node migration, lymphocyteactivation, effector T cell immune response, CD8<+> T and CD4<+> T cell responses, and memory T cells immune response.
Owner:INST OF BIOMEDICAL ENG CHINESE ACAD OF MEDICAL SCI
Vaccine compositions and methods of use
InactiveUS20150250872A1Provide protectionImprove responseSsRNA viruses negative-senseAntibacterial agentsDiseaseAdjuvant
The present disclosure provides vaccine compositions comprising at least one adjuvant and at least one antigen, wherein the adjuvant is a cationic lipid. The disclosure also provides methods of treating a disease in a mammal, methods of preventing a disease in a mammal, and methods of effecting antigen cross presentation to induce a humoral immune response and a cellular immune response in a mammal utilizing the vaccine compositions. Cross presentation of various antigens can be achieved by formulating the specific antigens with cationic lipids possessing adjuvant properties.
Owner:PDS BIOTECH
Vaccibodies targeted to cross-presenting dendritic cells
InactiveCN104136039ASsRNA viruses negative-senseAntibody mimetics/scaffoldsDendritic cellBiochemistry
The present invention relates to recombinant fusion proteins targeted to dendritic cells and uses thereof. In particular, the present invention relates to fusion proteins comprising an antibody component and a targeting components, and uses of such fusion proteins to trigger immune responses.
Owner:UNIVERSITY OF OSLO
Application of crosslinked polyethylenimine as oncoprotein antigen vaccine vector
ActiveCN105012957APharmaceutical non-active ingredientsAntibody medical ingredientsDendritic cellCytotoxicity
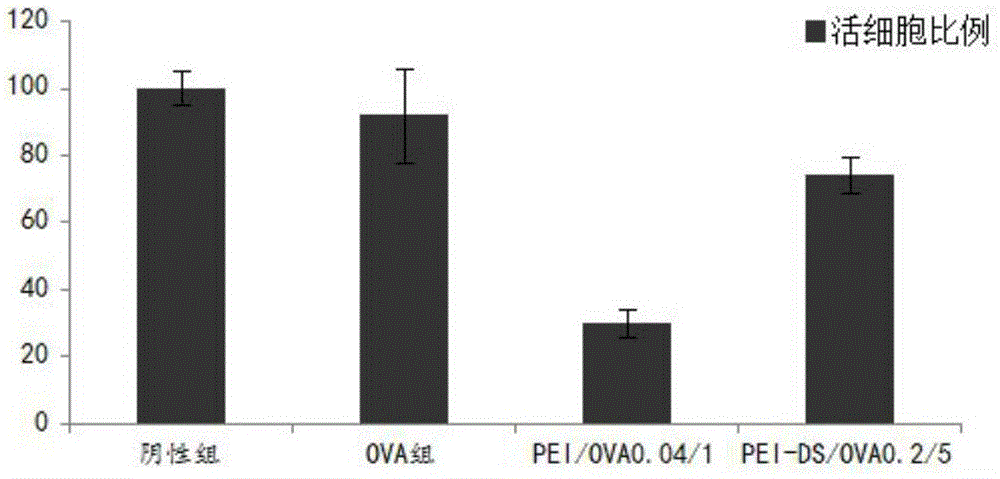
The present invention provides application of crosslinked polyethylenimine as an oncoprotein antigen vaccine vector, and overcomes the problem that protein antigen after penetration into the body can be degraded easily by enzyme, leading to weak immunogenicity of the vaccine. In an aqueous solution, the polymer material is capable of forming a complex antigen nanoparticle with antigen, in order to achieve loading of tumor protein antigen. The present invention uses RF33.70 as a target cell model, OVA as a model antigen, and C57BL / 6 mice bone marrow-derived dendritic cells as antigen cross presenting cells, and detects the antigen cross-presentation, cytotoxicity and in vivo anti-tumor effect of the complex nanoparticles formed by biodegradable PEI as the antigen vector and the OVA.
Owner:SHANGHAI JIAO TONG UNIV
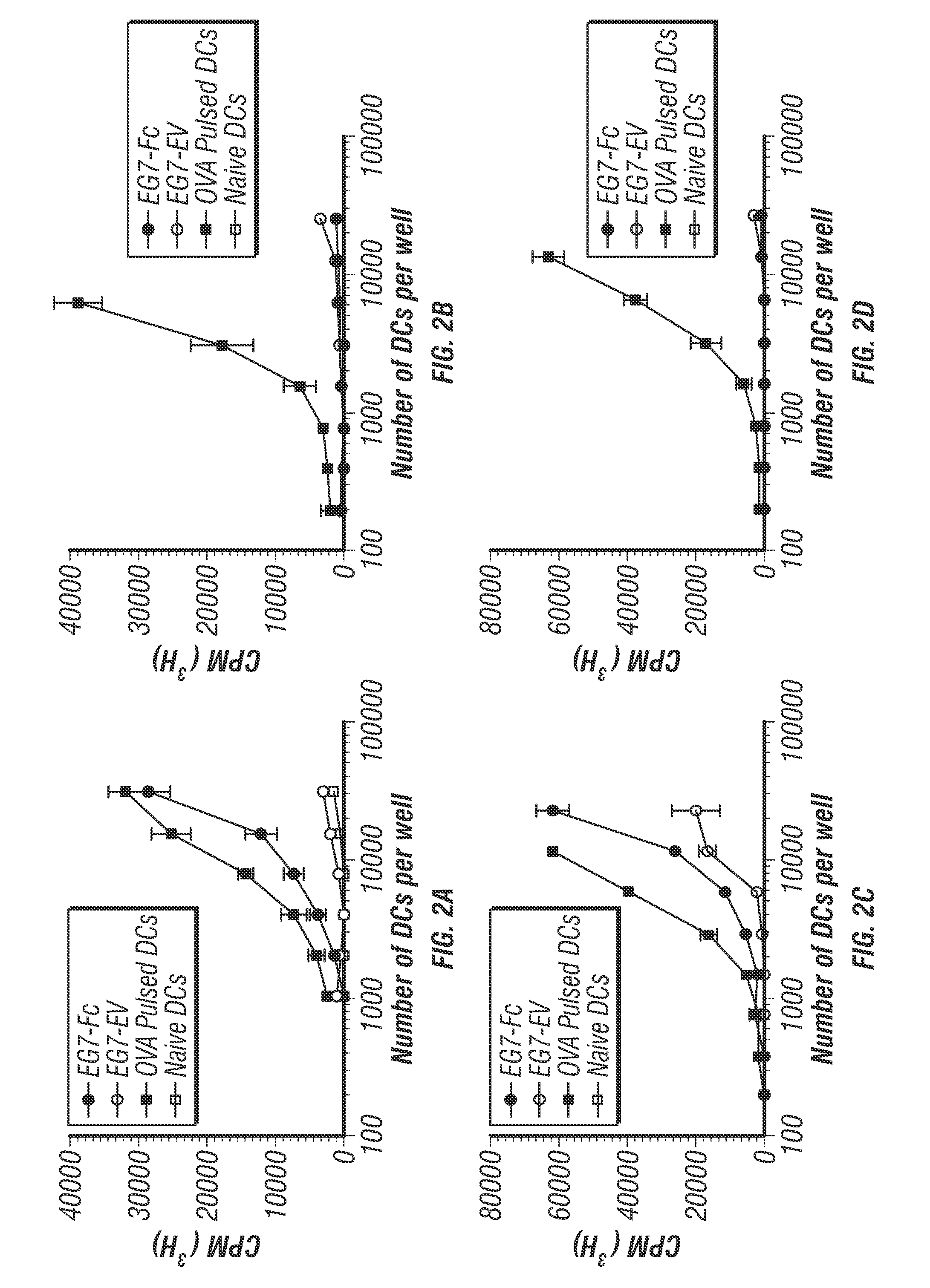
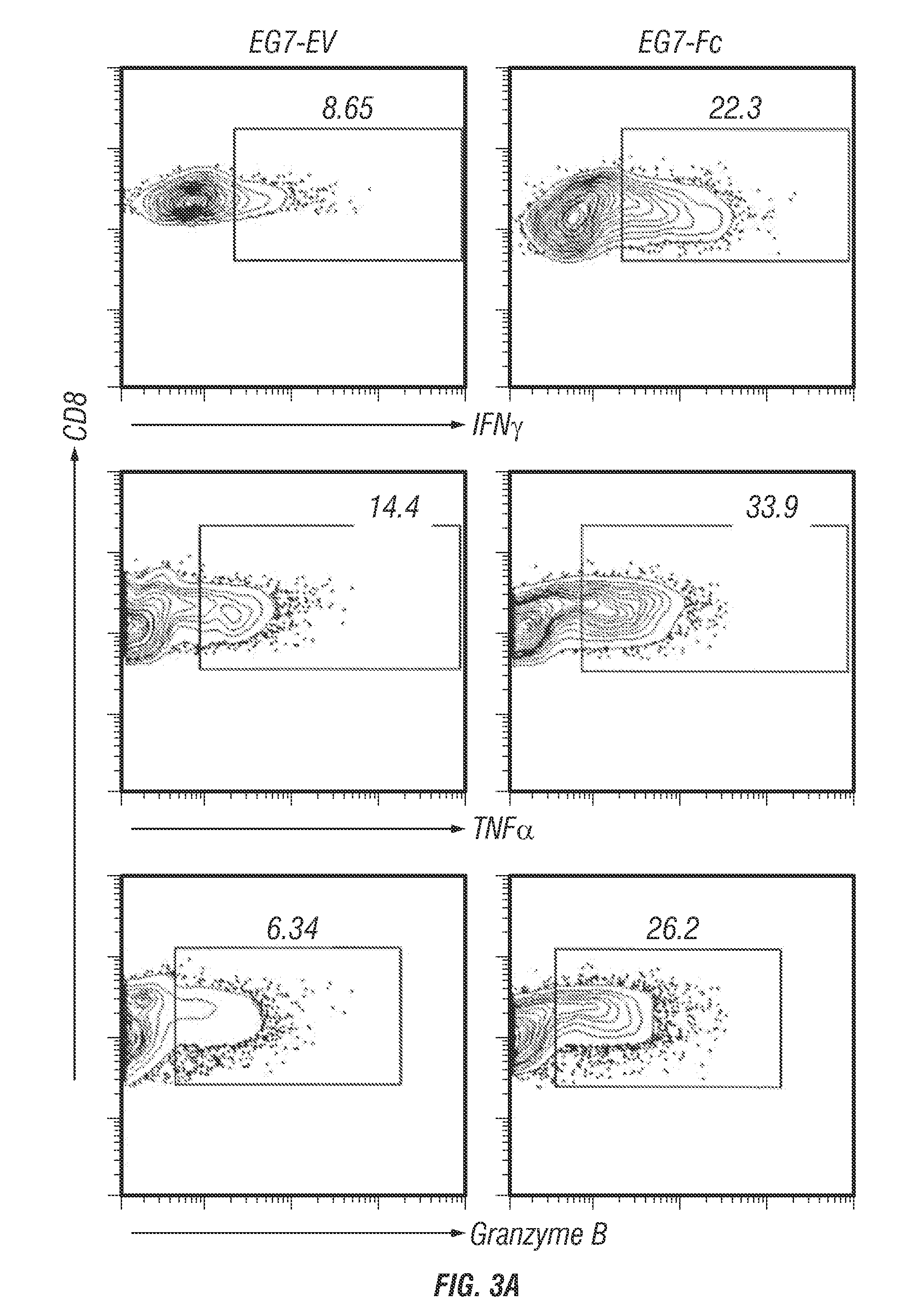
TUMORS EXPRESSING IgG1 Fc INDUCE ROBUST CD8 T CELL RESPONSES
InactiveUS20170007685A1Inhibit transferInduce primary tumor regressionGenetically modified cellsGenetic material ingredientsAntigenFc(alpha) receptor
A lymphoma cell line was engineered to express surface IgG1 Fc. These tumor cells were taken up rapidly by DCs, leading to enhanced cross-presentation of tumor-derived antigen to CD8 T cells. IgG1-Fc tumors failed to grow in vivo and prophylactic vaccination in an animal model resulted in rejection of unmanipulated tumor cells. Furthermore, IgG1-Fc tumor cells were able to slow the growth of an unmanipulated primary tumor when used as a therapeutic tumor vaccine. This demonstrates that engagement of Fc receptors by tumors expressing the Fc region of IgG1 can induce efficient and protective anti-tumor CD8+ T cell responses without prior knowledge of tumor-specific antigen.
Owner:UNITED STATES OF AMERICA +2
Clec9a-targeting affinity peptide WH peptide
ActiveCN105924501AImprove permeabilityEasy to synthesizePeptidesCarrier-bound antigen/hapten ingredientsAntigenIn vivo
The invention belongs to the technical field of tumor prevention and treatment and concretely relates to a Clec9a-targeting affinity peptide WH peptide and its preparation method and use. The peptide comprises 12 amino acids, has an amino acid sequence of WPRFHSSVFHTH and can be prepared through an Fmoc solid-phase synthesis method. The peptide can be used in tumor prevention and treatment. The peptide can be obtained through phage-displayed peptide library technology screening. The result of the research on an in-vivo and in-vitro foreign antigen cross presentation capacity and induction cases of specific CTL reaction shows that the WH peptide as a targeting carrier can well induce specific CTL response, improve IFN-gamma and related cell killing molecule expression quantity and improve tumor resistance. Through a computer simulated molecular docking technology, a possible docking area between the WH peptide and the Clec9a protein and action sites of the possible docking area can be researched. The peptide provides good reference and reference significance for related research.
Owner:ZHENGZHOU UNIV
Methods for Selecting Proteins that are Readily Presented as Antigens on Dendritic Cells
InactiveUS20080064048A1Blood/immune system cellsMaterial analysisDendritic cellProteasome degradation
The present inventors worked on elucidating the mechanism of antigen cross-presentation by dendritic cells, and revealed that foreign antigens taken up in dendritic cells are degraded by proteasomes after undergoing polyubiquitination. They discovered a positive correlation between the uptake amount of an antigenic protein by dendritic cells or the level of polyubiquitination and the level of antigen presentation of the antigenic protein. Based on these findings, methods were developed for predicting the intensity of cross-presentation of an anti genic protein by measuring the amount of antigenic protein taken up or polyubiquitinated protein. The use of these methods allows selecting antigenic proteins that are readily presented as antigens from among a number of proteins. The methods of the present invention enable suitable cancer immune vaccines to be provided through selection of more suitable cancer-specific proteins.
Owner:MEDICAL & BIOLOGICAL LAB CO LTD +1
Bacterial outer membrane vesicle carrier as well as preparation method and application thereof
ActiveCN112773901AFacilitate cross-presentationInduce specific immune responseBacteriaAntibody mimetics/scaffoldsDendritic cellAntiendomysial antibodies
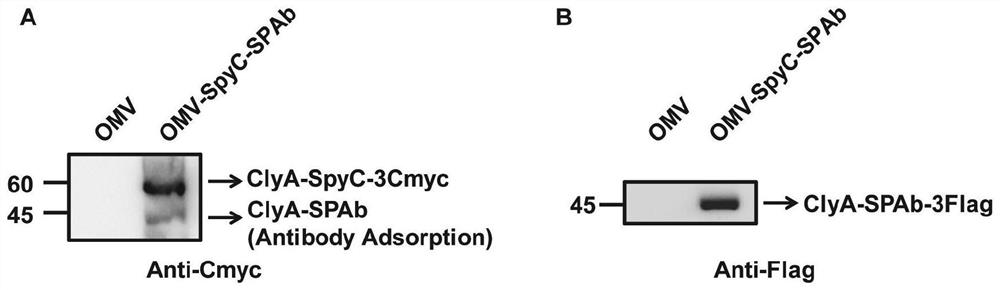
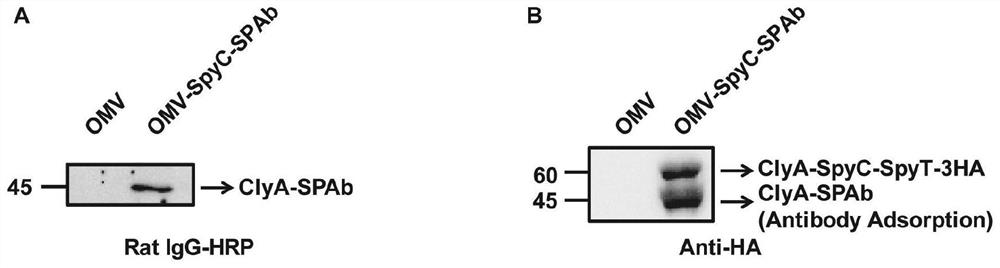
The invention provides a bacterial outer membrane vesicle carrier as well as a preparation method and application thereof. The invention provides a vaccine universal vector OMV-SpyC-SPAb which is constructed on the basis of a B structural domain SPAb of staphylococcus aureus protein A, is based on bacterial outer membrane vesicles and can target dendritic cells, the B structural domain SPAb of the staphylococcus aureus protein A is displayed on the bacterial outer membrane vesicles and can be rapidly combined with an antibody (such as an Anti-DEC205 antibody) of the targeted dendritic cells, and a novel OMV-OVA-DEC vaccine capable of targeting the dendritic cells and obviously enhancing antigen intake is prepared by using the SpyCatcher / SpyTag molecular glue to display the antigen on the surface of the bacterial outer membrane vesicles, so that the cross presentation of the dendritic cells on the antigen can be promoted, and the specific immune response can be induced, thereby achieving the anti-tumor purpose and having a wide application prospect.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
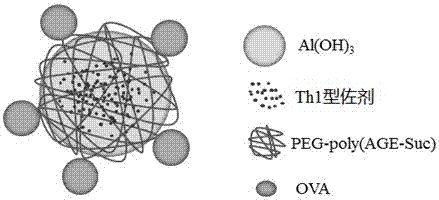
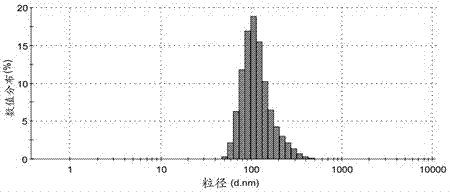
Vaccine carrier based on aluminum hydroxide nanoparticles
PendingCN107496915AReduce aluminum contentNo inflammationPharmaceutical non-active ingredientsImmunological disordersDendritic cellAluminium hydroxide
The invention relates to a vaccine carrier based on aluminum hydroxide nanoparticles. A PEG derivate material and an aluminum salt are compounded into nanoparticles. The vaccine carrier not only remains the property of powerful Th2 humoral immunologic adjuvant of the aluminum salt but also can be effectively in vivo transferred to drainage lymph gland and can be easily absorbed by dendritic cells (DC), can perform effective cross presentation, can induce cellular immunologic response and has strong Th1 immune response.
Owner:SICHUAN UNIV
Liposome preparation for tumor therapeutic vaccine and preparation method thereof
InactiveCN101816631AFree from excessive degradationRetained epitopeInorganic non-active ingredientsAntibody medical ingredientsCause specificLiposome
The invention relates to a preparation and a preparation method thereof, which belong to the technical field of anti-tumor medicament. The preparation comprises the following components in percentage by weight: 0.3 to 3 percent of hydrocarbonate or carbonate, 0.0001 to 5 percent of tumor proteantigen, 0.1 to 10 percent of lipid and the balance of water. The preparation can promote the cross-presentation of protein or polypeptide antigen loaded by the preparation so as to cause specific cellular immunity and fulfill the purpose of curing tumor or virus infection.
Owner:SHANGHAI JIAO TONG UNIV
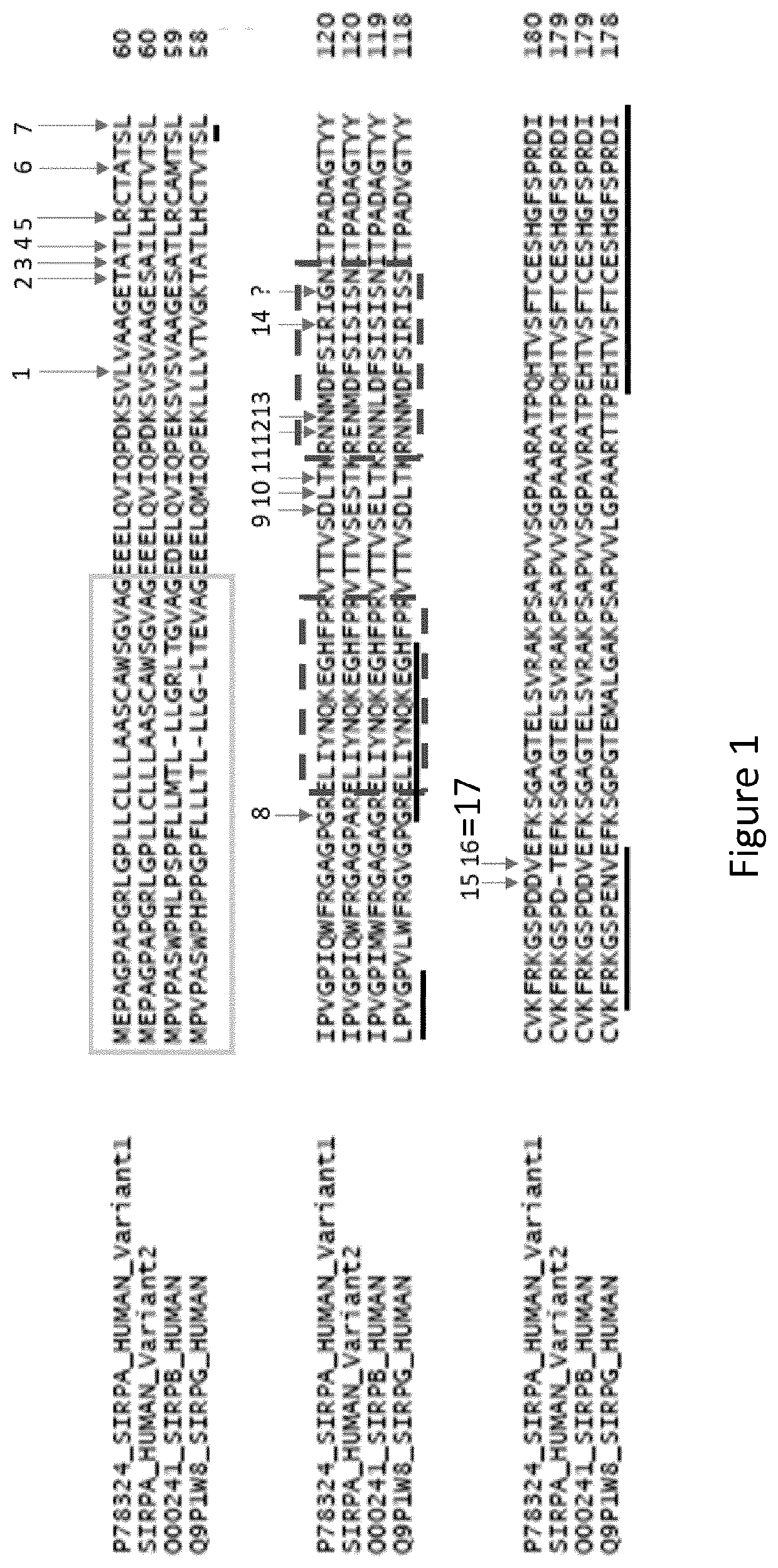
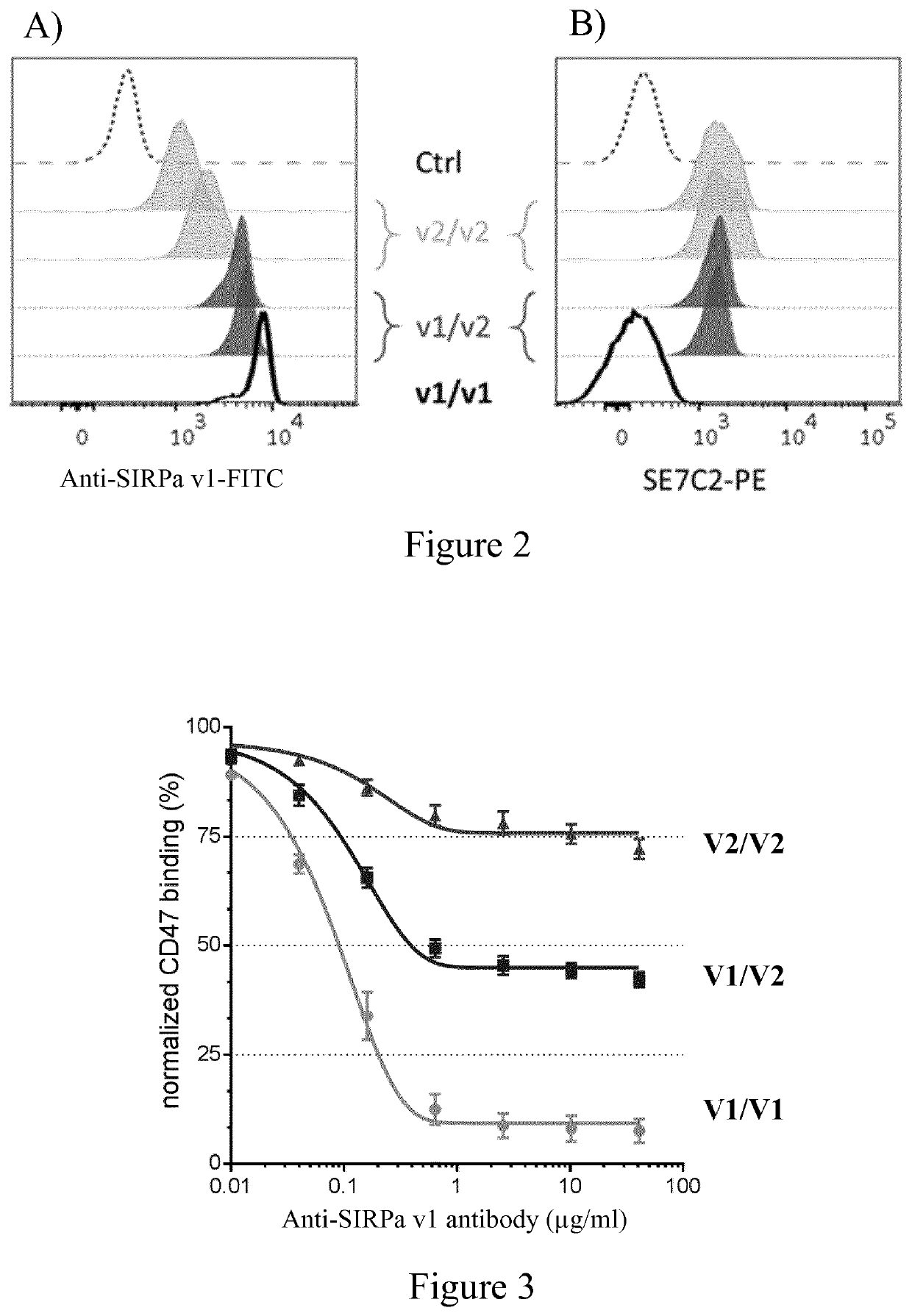
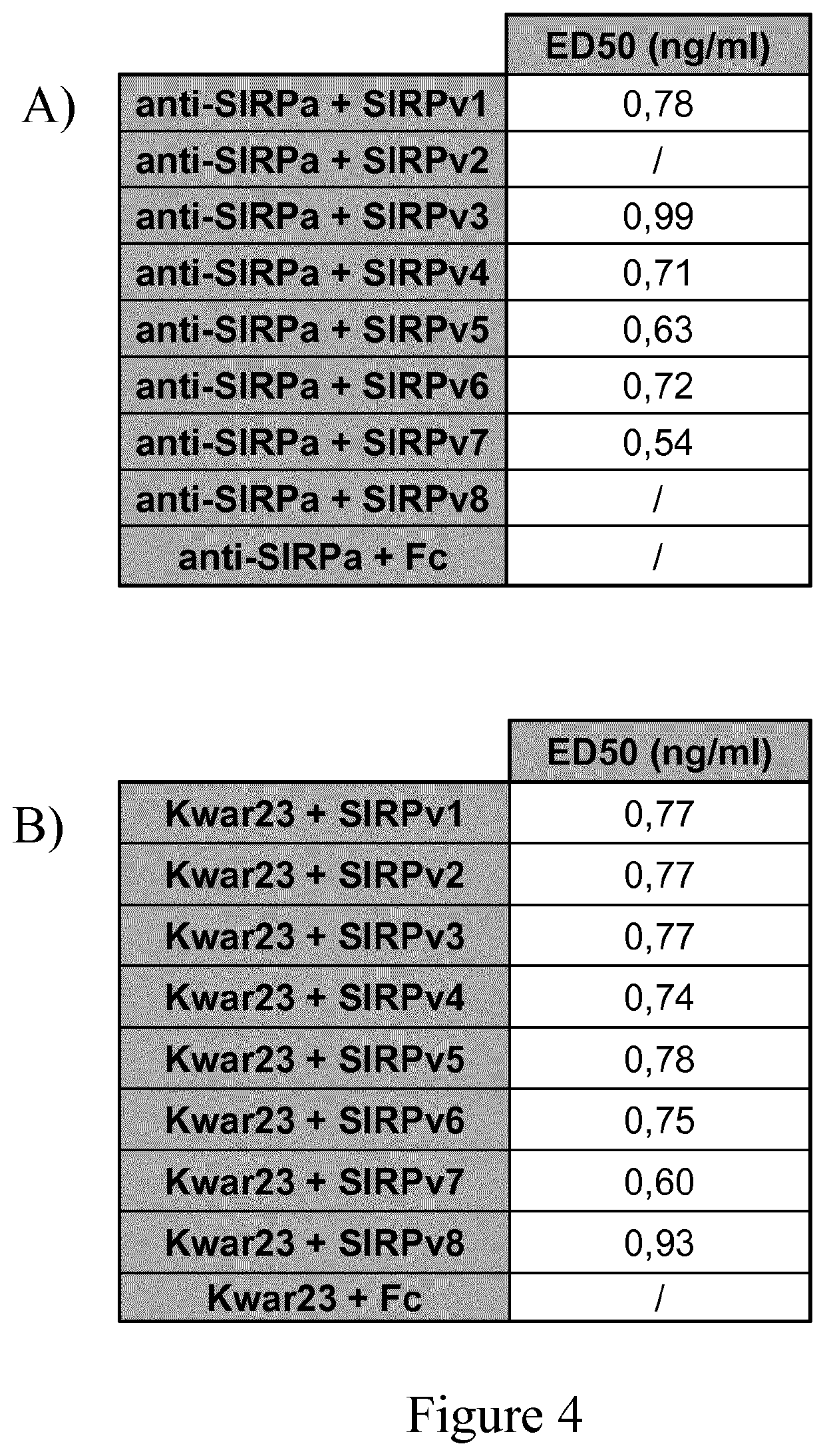
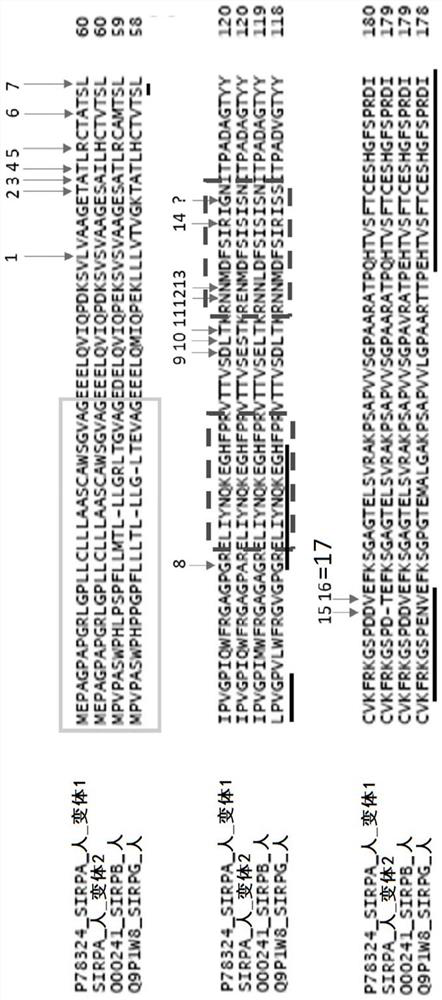
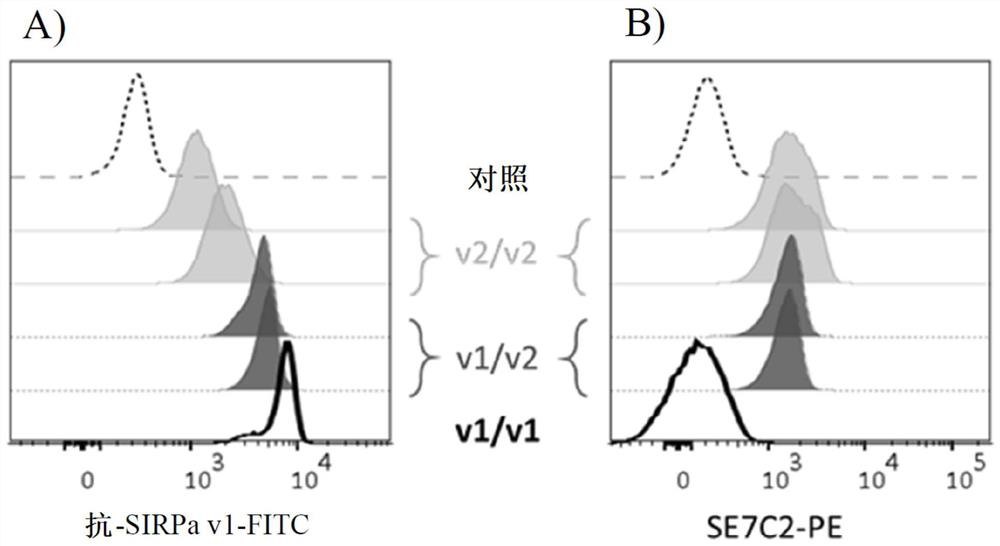
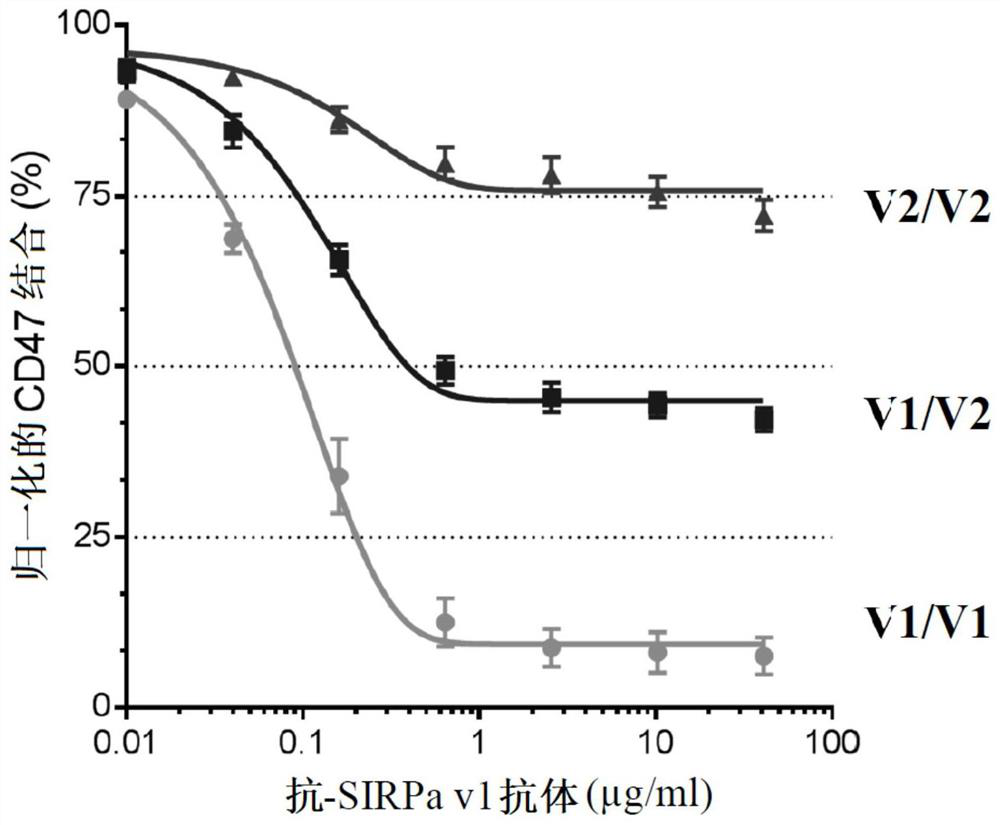
Use of Anti-human sirpa v1 antibodies and method for producing Anti-sirpa v1 antibodies
PendingUS20210040206A1Improve solubilityTissue penetrationImmunoglobulin superfamilyImmunoglobulins against cell receptors/antigens/surface-determinantsDiseaseAntiendomysial antibodies
The invention is in the field of immunotherapy. The present invention provides antibodies useful in therapeutic and diagnostic applications targeting human SIRPa, said antibodies enhancing the cross-presentation of antigens to T cells. The invention also provides antibodies against specific isoforms of SIRP a and able to disrupt the interaction between those isoforms of SIRP a and human CD47 for use in treating or preventing a disease, or diagnostic applications, and methods for producing and / or selecting anti-human SIRPa antibodies that bind with different affinities to various isoforms of human SIRP members.
Owner:OSE IMMUNOTHERAPEUTICS
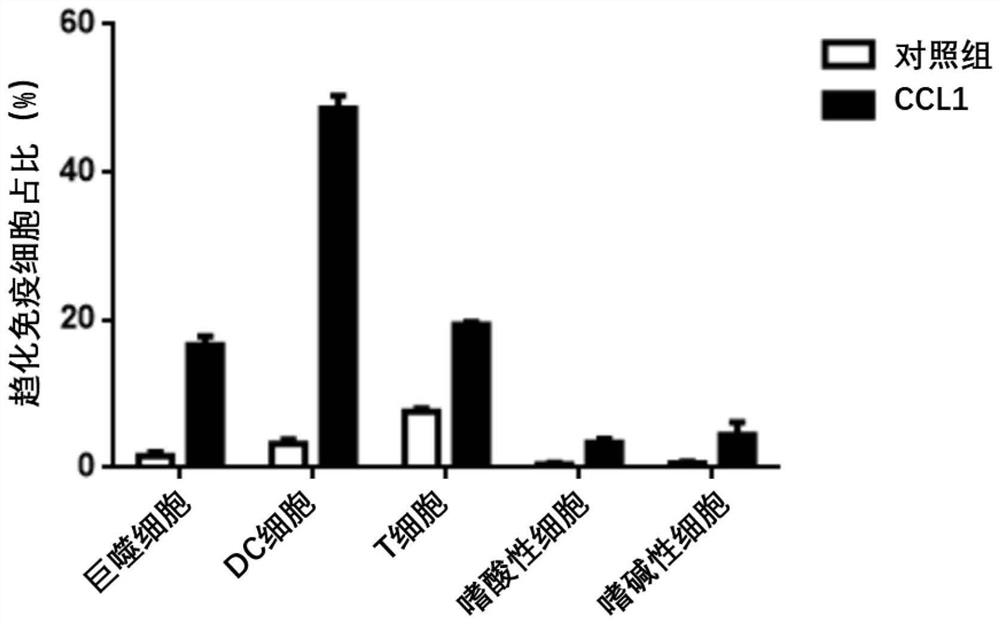
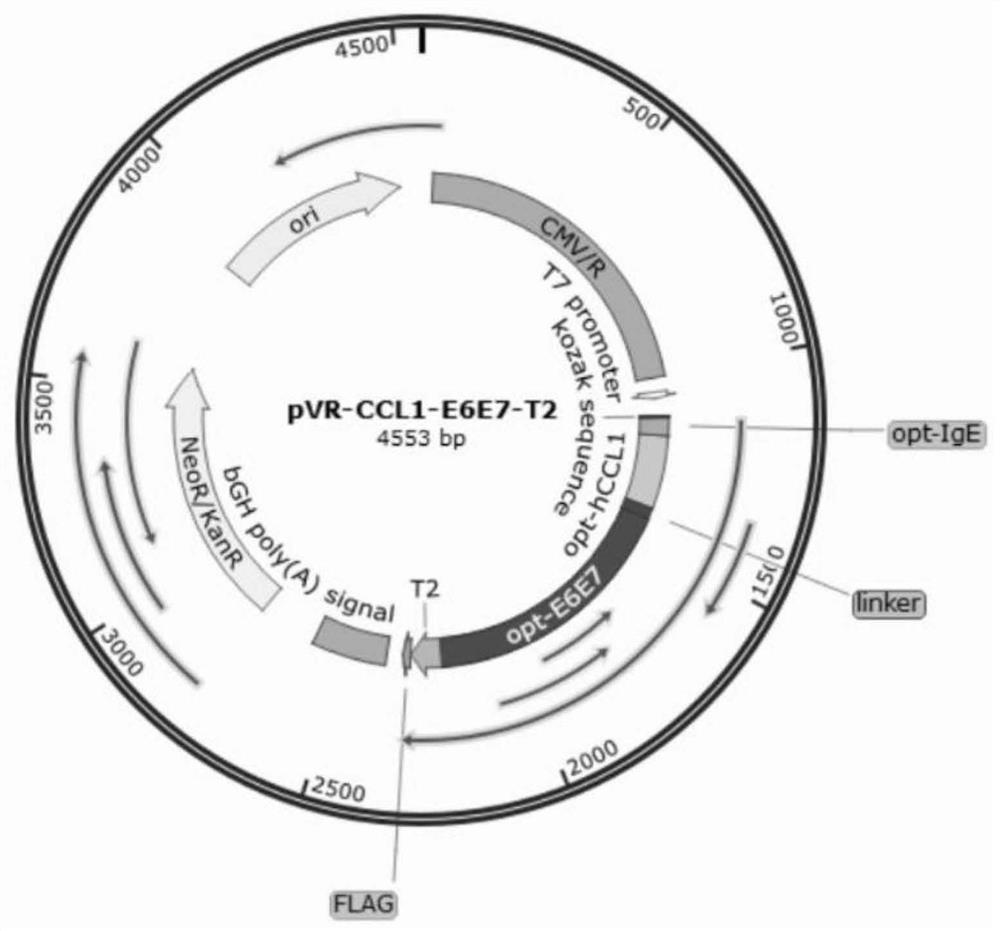
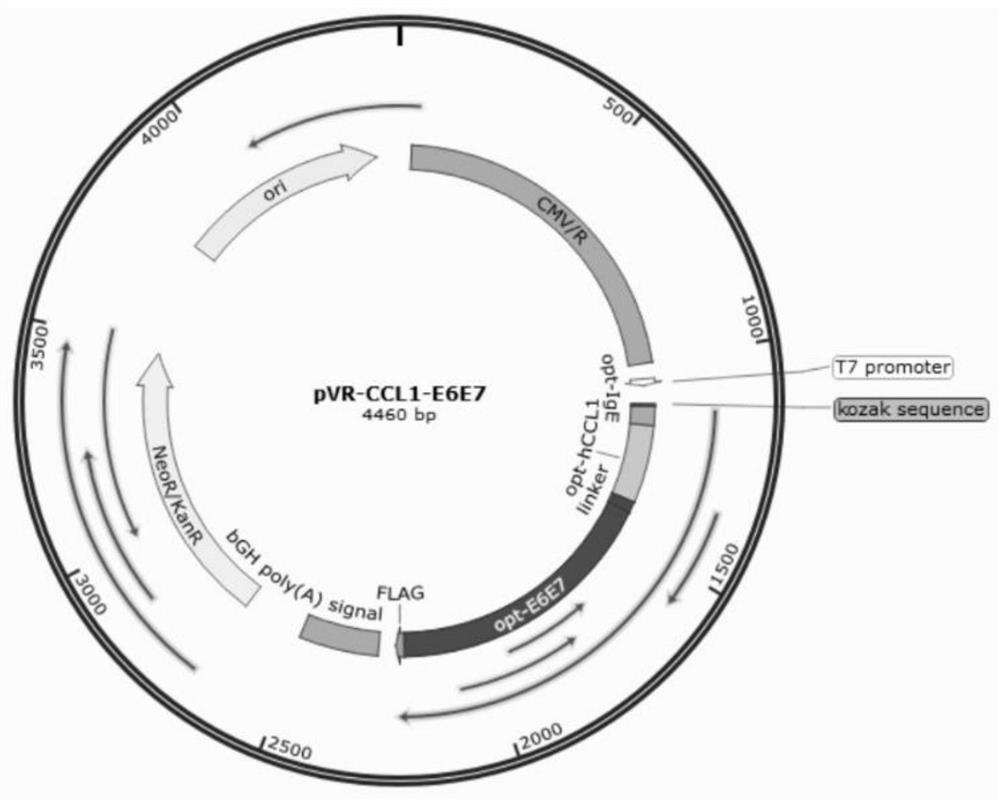
Fusion protein containing CCL1, preparation method and application
InactiveCN114573716AStrong immune boosting effectTrigger immune responsePolypeptide with localisation/targeting motifChemokinesDiseaseReceptor
The invention discloses a CCL1-containing fusion protein, a preparation method and application, the fusion protein sequentially comprises an IgE signal peptide, CCL1, a linker and an antigen from an N terminal to a C terminal, or the IgE signal peptide, CCL1, the linker, the antigen and a T2 fragment, and the amino acid sequence of the T2 fragment is shown as SEQ ID NO: 4. According to the invention, different antigen proteins are transferred and crossly presented to the surface of the DC cell by utilizing the chemotactic binding capacity of CCL1 and surface receptors of immune cells such as the DC cell, so that the efficiency of phagocytosis, processing and presentation of various antigen proteins by the DC cell is improved, and the effect of preventing and treating related diseases is improved. Experiments prove that the T2 sequence disclosed by the invention has a very strong immune enhancement effect, and can further excite body fluid and cellular immune response in the process of promoting antigen presentation, so that the effect of inhibiting growth of related tumors is finally achieved.
Owner:保定诺未科技有限公司
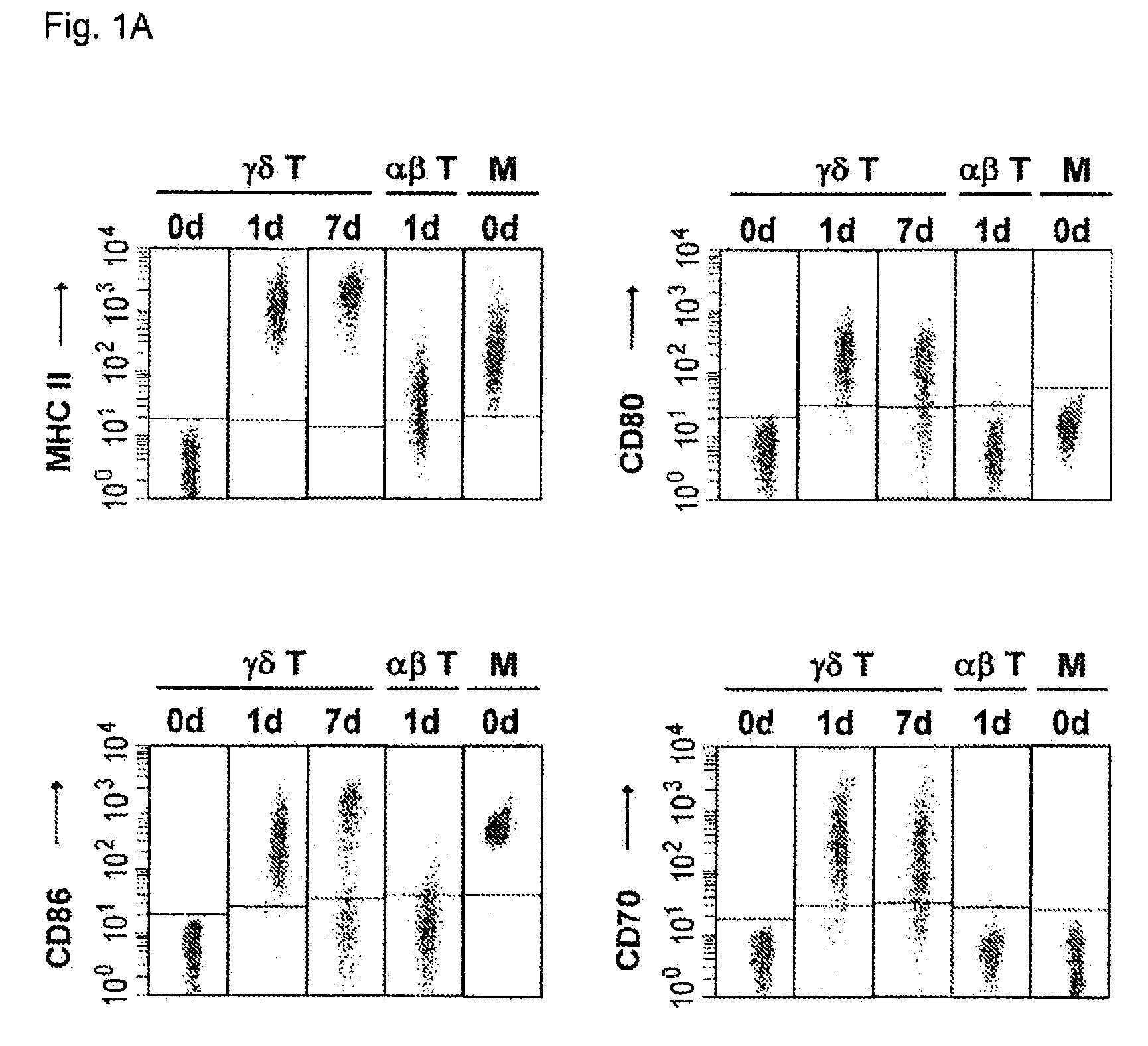
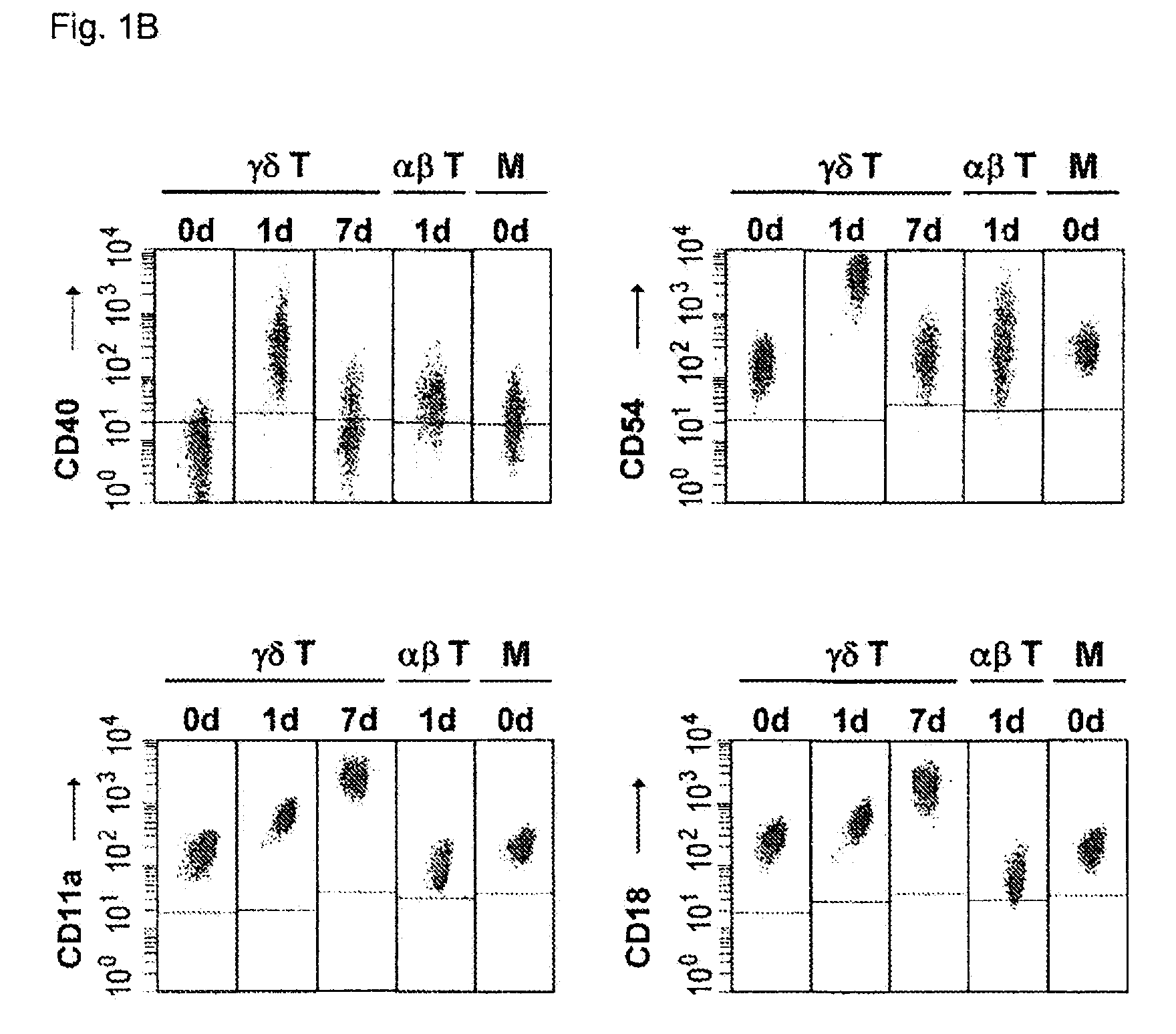
Preparation of antigen-presenting human γδ T cells and use in immunotherapy
InactiveUS8338173B2Improve purification effectEfficient workBiocideMammal material medical ingredientsAbnormal tissue growthDendritic cell
Cytotoxic αβ T cells form an essential component in immunity to infections and tumors, and are also implicated in host defense against these challenges. The present disclosure demonstrates the ability of activated γδ T cells to cross-present exogenous antigens to CD8+αβ T cells, a process previously thought to be mediated best by dendritic cells. In particular, the present disclosure provides a method for cross-presentation of antigen derived from tumor cell or microbial organisms such as viruses, bacteria, yeasts, parasites, and the like, or from cells infected with such organisms, to a CD8+αβ T cell. Still further, the present disclosure provides a method for treatment of a tumor or a chronic or recurrent infectious disease, comprising delivering an antigen-presenting autologous γδ T cell population above into a patient requiring such treatment. Still yet further, a method is described for preparing a peptide-specific effector T cell.
Owner:UNIV COLLEGE CARDIFF CONSULTANTS LTD
Varicella-zoster virus vaccine and application thereof
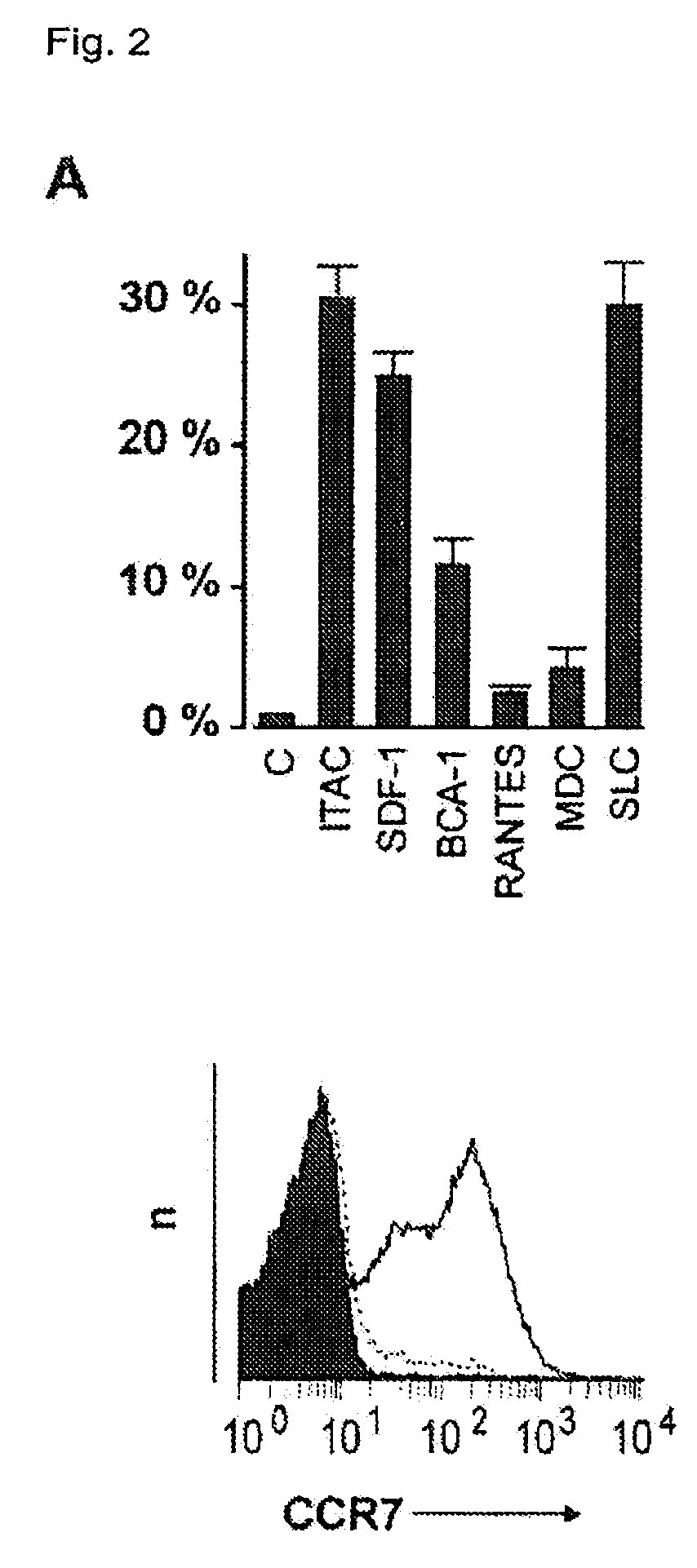
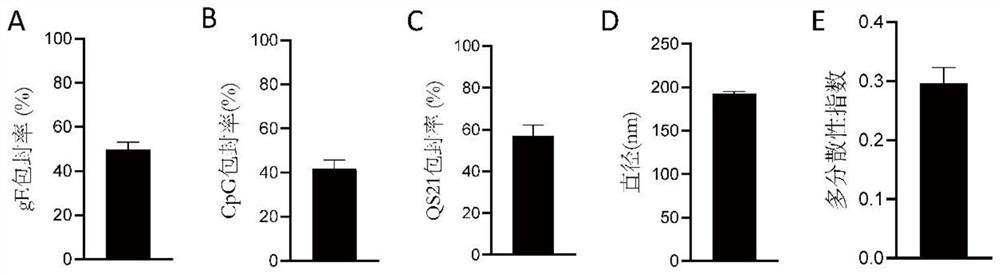
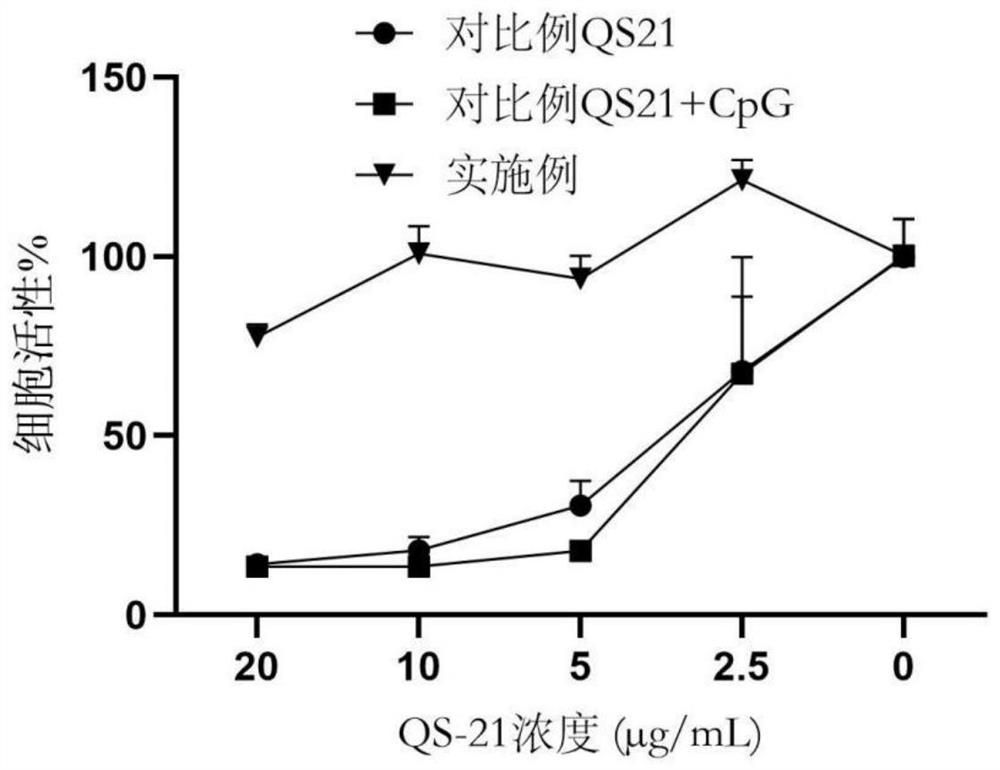
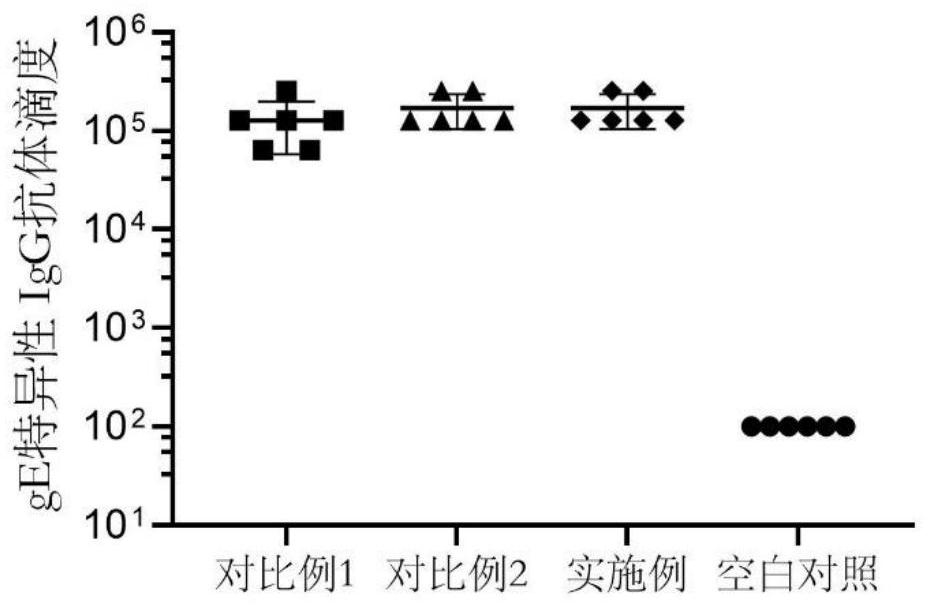
PendingCN114767844AImprove securityEnhance immune responseViral antigen ingredientsAntiviralsCholesterolHerpes zoster virus
The invention provides a varicella-zoster virus vaccine and application thereof, and belongs to the technical field of vaccines. The varicella-herpes zoster virus vaccine comprises liposome nano-particles, herpes zoster virus glycoprotein E and an adjuvant, wherein the herpes zoster virus glycoprotein E and the adjuvant are entrapped in the liposome nano-particles; the adjuvant comprises triterpenoid saponin. In the invention, after the herpes zoster virus glycoprotein E (gE) is wrapped by the liposome nanoparticles, antigen presenting cell phagocytosis and efficient antigen delivery can be effectively promoted, and sustained release of the vaccine is realized to continuously stimulate a body to generate specific cellular immune response aiming at VZV-gE. The triterpenoid saponin used in the invention can effectively realize cross presentation of antigen herpes zoster virus glycoprotein E and induce antigen-specific cellular immune response. Moreover, cholesterol rich in the lipid nanoparticles can effectively neutralize the cytotoxicity of the triterpenoid saponin, so that the safety of the vaccine is ensured.
Owner:TAIZHOU BIVO BIOTECH CO LTD
Use of Anti-human sirpa v1 antibodies and method for producing Anti-sirpa v1 antibodies
PendingCN112105646AImmunoglobulin superfamilyImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenDisease
The invention is in the field of immunotherapy. The present invention provides antibodies useful in therapeutic and diagnostic applications targeting human SIRPa, said antibodies enhancing the cross-presentation of antigens to T cells. The invention also provides antibodies against specific isoforms of SIRP a and able to disrupt the interaction between those isoforms of SIRP a and human CD47 for use in treating or preventing a disease, or diagnostic applications, and methods for producing and / or selecting anti-human SIRPa antibodies that bind with different affinities to various isoforms of human SIRP members.
Owner:OSE IMMUNOTHERAPEUTICS
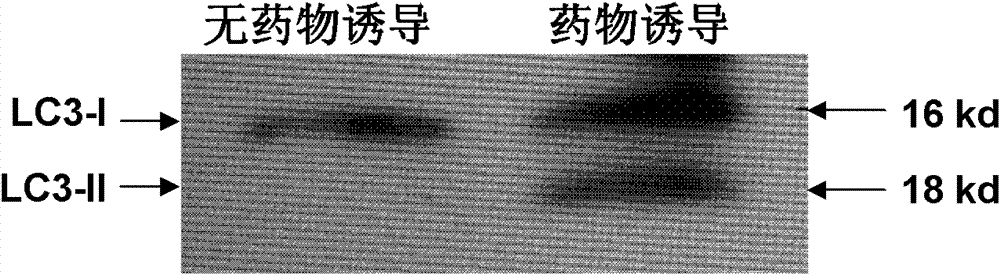
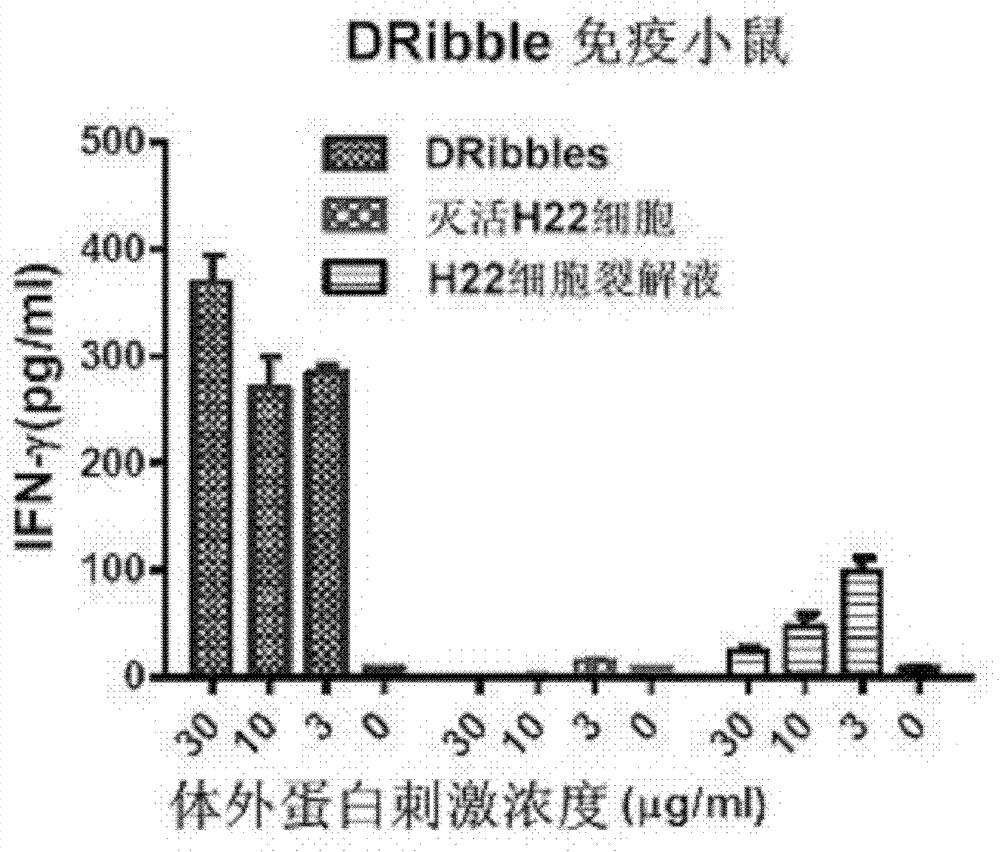
Application of H22 liver cancer cell autophagosome to preparation of liver cancer therapeutic vaccine
ActiveCN102430118BConform to the characteristicsEffective recruitmentTumor/cancer cellsAntibody medical ingredientsOncologyTumor antigen
The invention discloses application of H22 liver cancer cell autophagosome DRibbles to the preparation of a liver cancer therapeutic vaccine. Liver cancer is one of the most common malignant tumors in China and can be effectively prevented by developing a liver cancer vaccine; and years of research results prove that tumor cell autophagosome is one of effective vectors for tumor antigen cross-presentation. The application comprises a core technology for extracting the autophagosome DRibbles from human liver cancer cells and proves that the autophagosome can effectively induce anti-tumor immune response of organisms and has a good biotherapy prospect.
Owner:SOUTHEAST UNIV
Preparation method and application of antigen and adjuvant co-delivery nano vaccine based on protamine as carrier
ActiveCN112516297AImprove transfer efficiencyImprove stabilityCancer antigen ingredientsMacromolecular non-active ingredientsAdjuvantAntineoplastic Immunotherapeutic
The invention provides an antigen and adjuvant co-delivery nano vaccine based on protamine as a carrier. The nano vaccine comprises a carrier, an antigen and an adjuvant, wherein the carrier is protamine sulfate; The invention also provides a preparation method and application of the vaccine. The nano vaccine prepared by the invention can effectively improve uptake efficiency of antigen presentingcells on antigens and adjuvants, efficiently stimulate maturation of the antigen presenting cells, assist the antigens in realizing cross presentation, significantly improve in-vivo transfer efficiency of the antigens and the adjuvants, stimulate a body to produce high-efficiency humoral immunity and cellular immunity, and at the same time produce an immune memory effect. In-vivo treatment is carried out on mouse subcutaneous melanoma, and it is proved that the nano vaccine has a good anti-tumor immunotherapy effect. The nano vaccine is simple in structure, convenient to prepare and excellentin immunotherapy performance, and has good application potential.
Owner:WEIFANG MEDICAL UNIV
Tumor vaccine treatment method
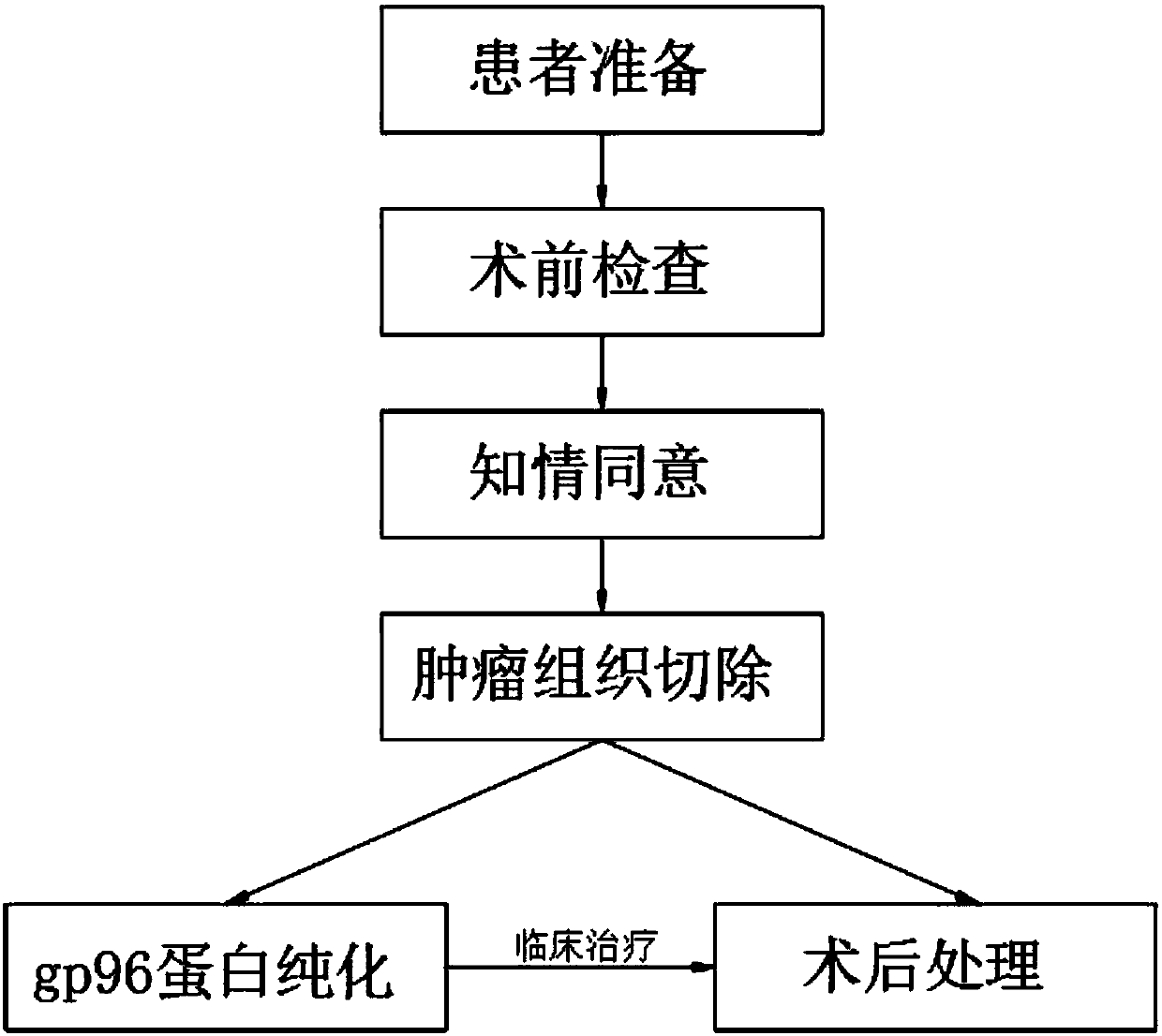
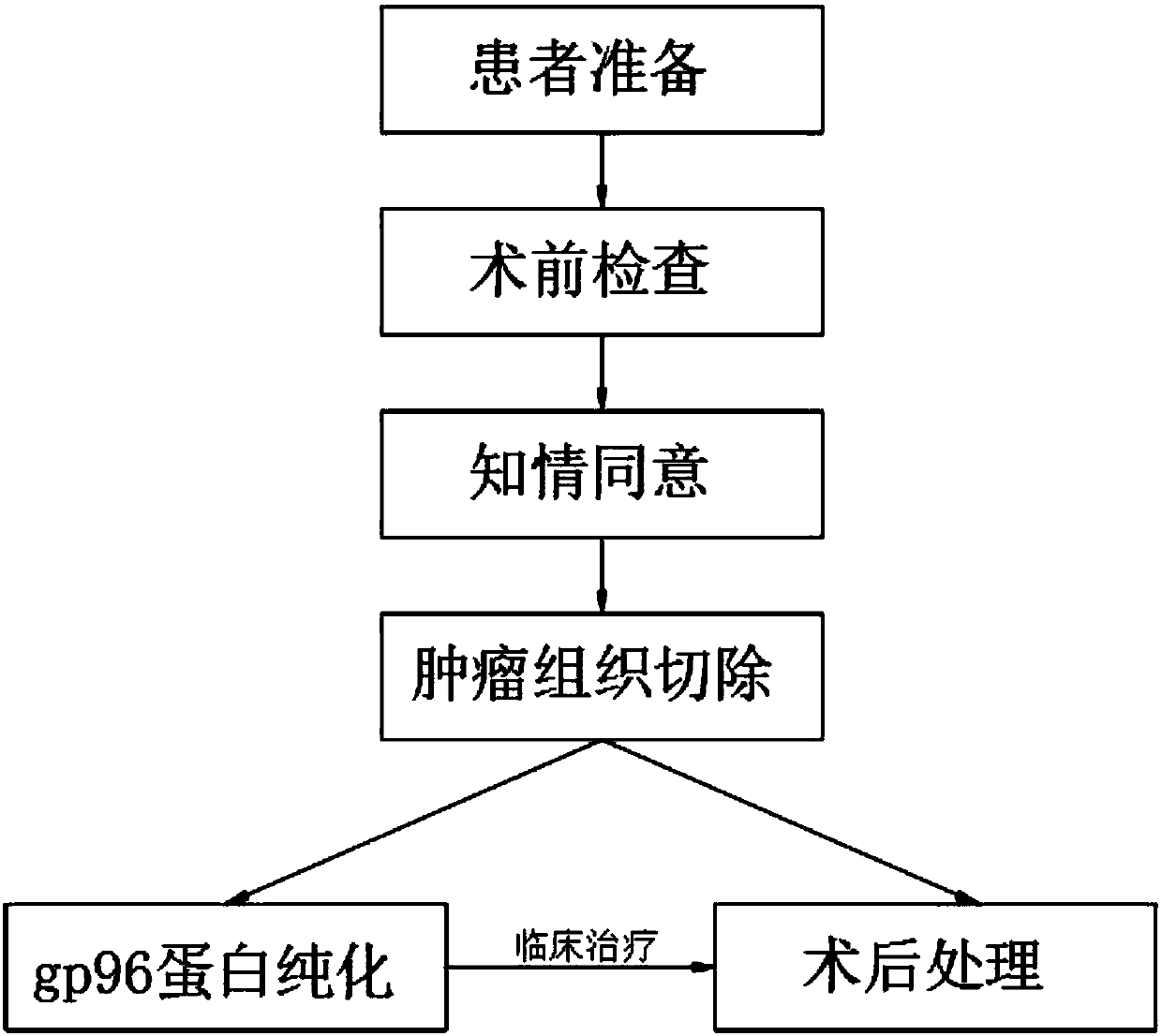
InactiveCN109833473AWide variety of sourcesEasy accessAntibody medical ingredientsAntineoplastic agentsAntigenCytokine
The invention discloses a tumor vaccine treatment method, which comprises the following steps: patient preparation, preoperative examination, informed agreement, tumor tissue resection, postoperativetreatment, gp96 protein purification and clinical treatment. According to an immunization procedure, the patient is subjected to administered subcutaneous or intradermal injection with a tumor autologous gp96 vaccine, and gp96 interacts with heat shock protein receptors (CD91, CD36, SR-A, Lox-1, TLR2 and TLR4) on the surface of antigen presenting cells, the maturation and activation of antigen-presenting cells can be promoted, which are realized by up-regulating of cell surface molecules and secreting of cytokines and chemokines, the adjuvant properties of gp96 and its bonded tumor-derived polypeptides provide an ideal environment for cross-presenting MIHC I and MHC II restricted polypeptides, tumor autologous gp96 activates tumor cells and micrometastases of tumor cell-specific CD4<+> helper T cells and cytotoxic CD8<+> T cells, the method has good immunization characteristic, and the treatment effect is better than that of the operation and chemotherapy.
Owner:杨涛
Compositions and methods for enhancing immunogenic cross-presentation of tumor antigens
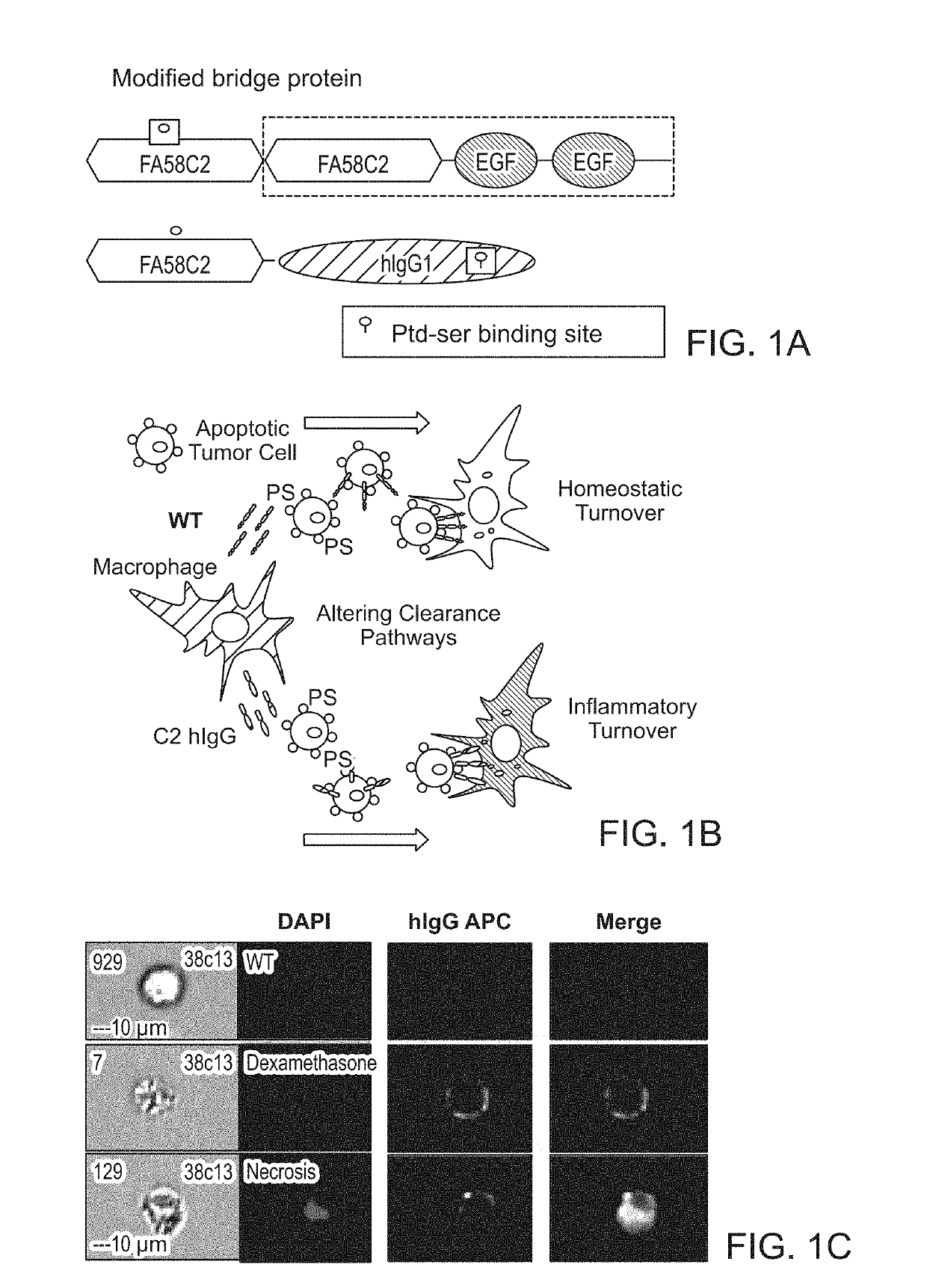
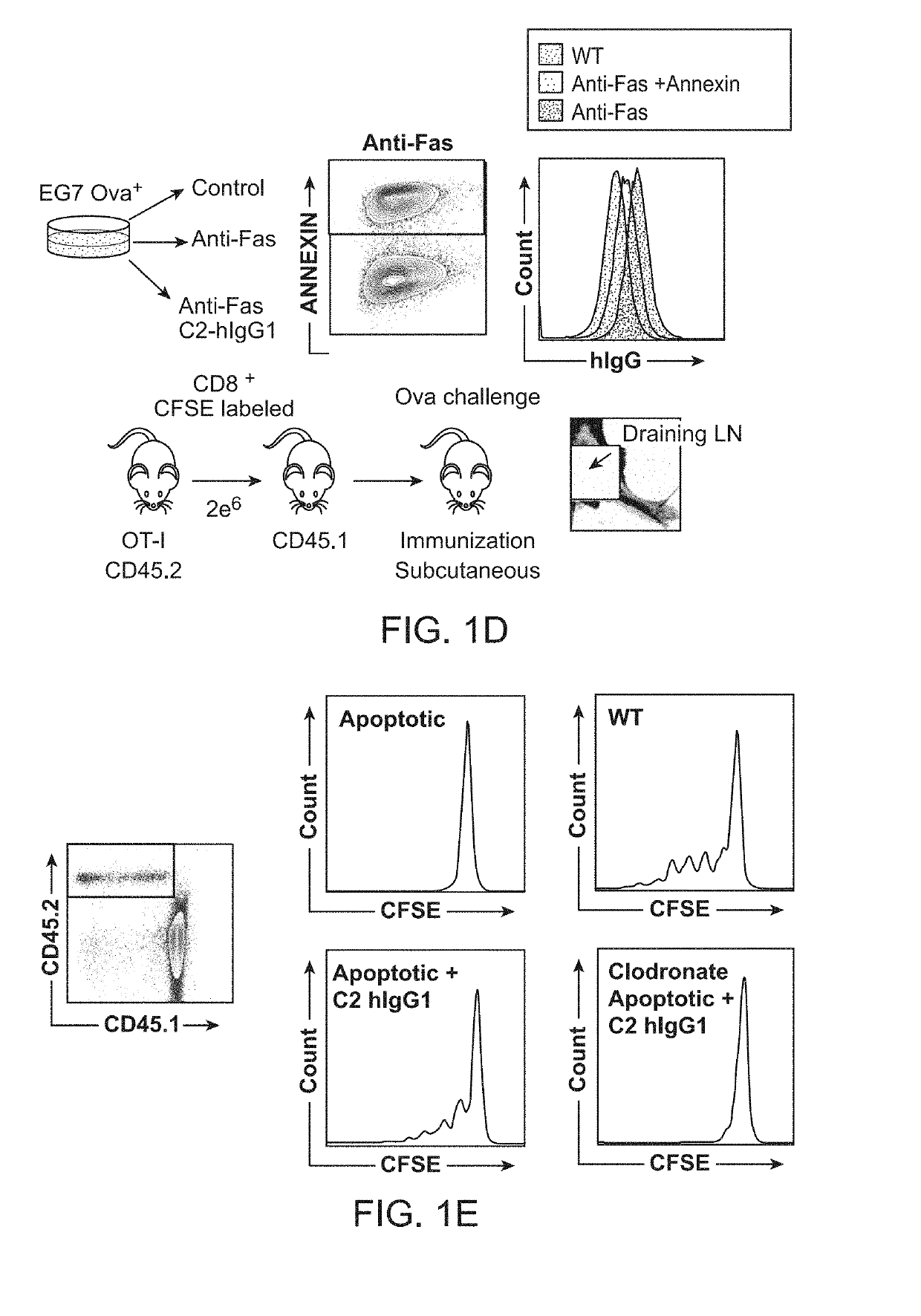
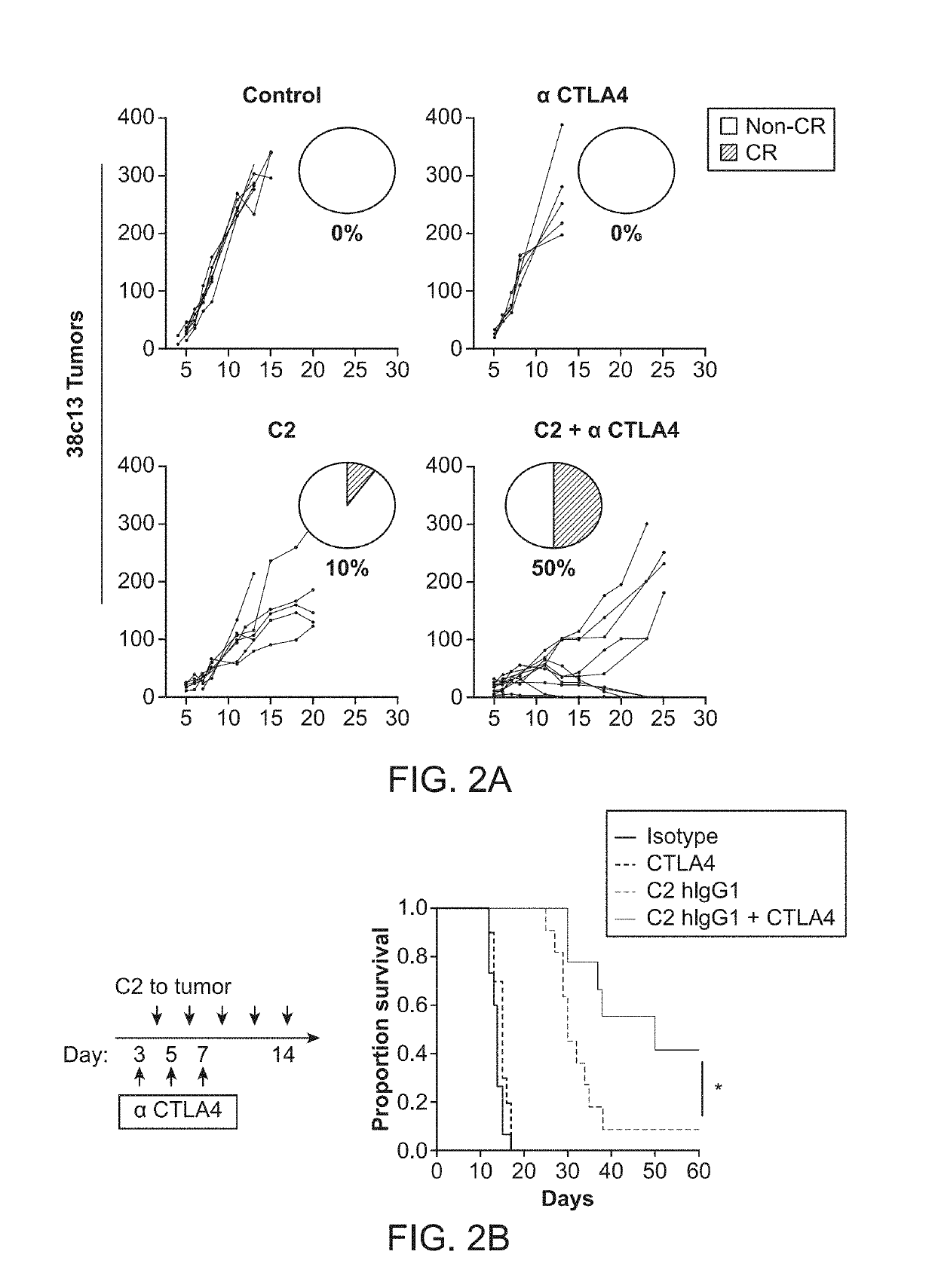
ActiveUS20190218260A1Promote inflammatory turnoverImproving immunogenicityPeptide/protein ingredientsAntibody mimetics/scaffoldsDiseaseApoptosis
Cell loss by apoptosis is a common feature in certain conditions, including cancer. Dying tumor cells induce immune tolerance within the tumor microenvironment largely through highly conserved homeostatic clearance programs that are designed to restore tissue homeostasis and contribute to the formation of an immunosuppressive niche. The translocation of phosphatidylserine (PS) on cellular membranes, during the initial phases of apoptosis, functions as a recognition and removal signal that limits the immunogenicity of cell death. To remove inhibitory signals in the homeostatic clearance pathway a fusion protein comprising a phosphatidylserine binding domain and an immunostimulatory domain can restore immune responses to dead tumor cells in antigen cross presentation assays and promotes recruitment and retention of tumor antigen specific immune effector cells into tumors. These effects combine to elicit anti-tumor immunity, improve responses to immune checkpoint inhibitors, and enhance the effectiveness of adoptive T cell transfers using engineered T cells.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Vaccibodies targeted to cross-presenting dendritic cells
InactiveUS20140234316A1SsRNA viruses negative-senseAntibody mimetics/scaffoldsEpidermal Dendritic CellsDendrite
The present invention relates to recombinant fusion proteins targeted to dendritic cells and uses thereof. In particular, the present invention relates to fusion proteins comprising an antibody component and a targeting components, and uses of such fusion proteins to trigger immune responses.
Owner:UNIVERSITY OF OSLO
Endoplasmic reticulum Golgi apparatus targeting micromolecule, conjugate and application of endoplasmic reticulum Golgi apparatus targeting micromolecule
ActiveCN114163420AEfficient amplificationImprove cross-presentationPeptidesCarrier-bound antigen/hapten ingredientsReticulum cellIncreased inflammatory response
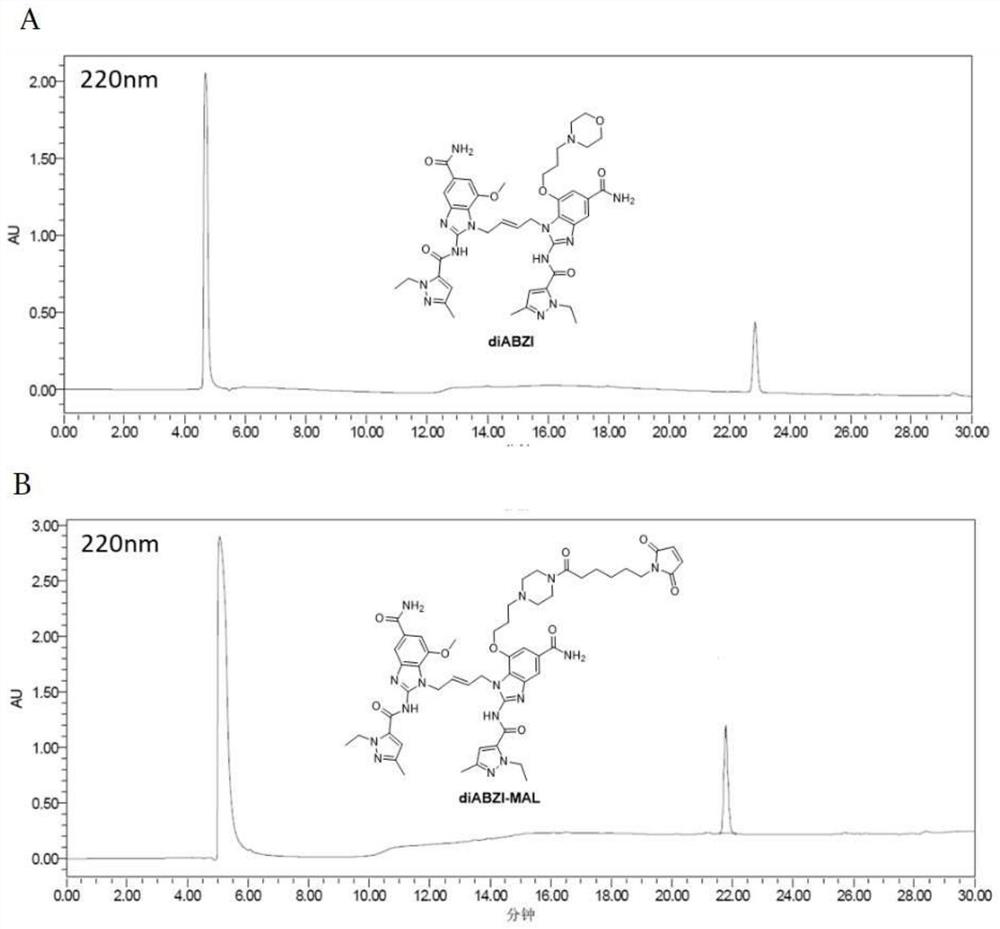
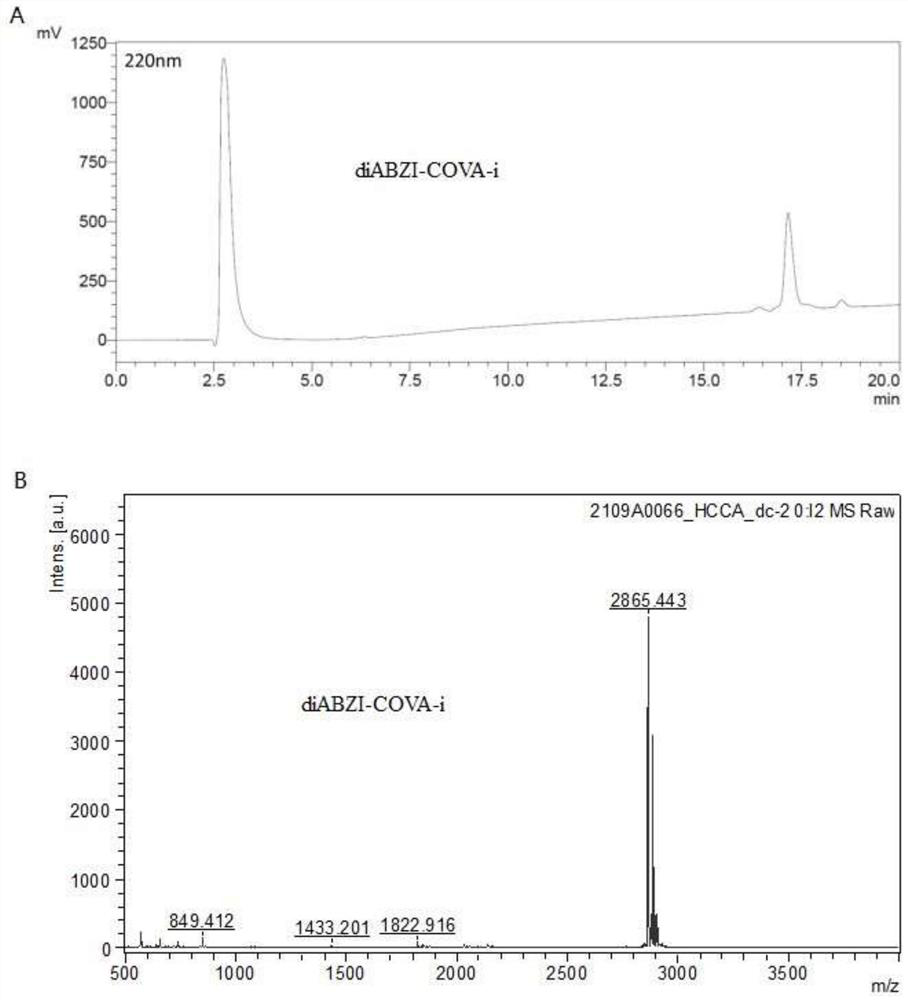
The invention discloses an endoplasmic reticulum Golgi apparatus targeting micromolecule. The structural formula of the endoplasmic reticulum Golgi apparatus targeting micromolecule is as shown in formula I, the invention also provides an endoplasmic reticulum Golgi apparatus targeting micromolecule conjugate, and the structural formula of the conjugate is as shown in the formula III. The invention also provides an application of the endoplasmic reticulum Golgi apparatus targeting micromolecule and the conjugate in preparation of an STING protein agonist, or in improvement of an antigen cross presentation level, or in improvement and enhancement of antigen targeting endoplasmic reticulum Golgi apparatus, or in preparation of a drug for inducing CD8 + T immune response, or in preparation of an immunotherapy drug. According to the endoplasmic reticulum Golgi apparatus targeting micromolecule and the conjugate provided by the invention, good connection between the endoplasmic reticulum Golgi apparatus targeting micromolecule and an antigen can be realized, the binding force of a bis-benzopyrimidine skeleton to STING protein is maintained, systemic inflammatory response caused by excessive diffusion of an STING agonist diABZI is reduced, the antigen targeting endoplasmic reticulum Golgi apparatus at a subcellular level is enhanced, and the immunogenicity of the antigen is improved. The antigen cross presentation level is improved, DC cell maturation can be promoted, and CD8 + T immune response is efficiently induced.
Owner:THE FIRST AFFILIATED HOSPITAL OF SUN YAT SEN UNIV
Use of a cross-linked polyethyleneimine as a tumor protein antigen vaccine carrier
ActiveCN105012957BPharmaceutical non-active ingredientsAntibody medical ingredientsCross-linkDendritic cell
The present invention provides application of crosslinked polyethylenimine as an oncoprotein antigen vaccine vector, and overcomes the problem that protein antigen after penetration into the body can be degraded easily by enzyme, leading to weak immunogenicity of the vaccine. In an aqueous solution, the polymer material is capable of forming a complex antigen nanoparticle with antigen, in order to achieve loading of tumor protein antigen. The present invention uses RF33.70 as a target cell model, OVA as a model antigen, and C57BL / 6 mice bone marrow-derived dendritic cells as antigen cross presenting cells, and detects the antigen cross-presentation, cytotoxicity and in vivo anti-tumor effect of the complex nanoparticles formed by biodegradable PEI as the antigen vector and the OVA.
Owner:SHANGHAI JIAOTONG UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com