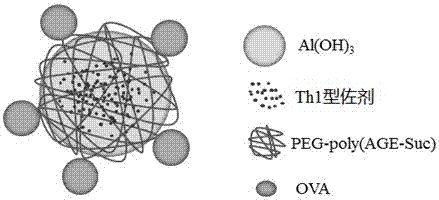
Vaccine carrier based on aluminum hydroxide nanoparticles
A technology of aluminum hydroxide and vaccine carrier, which is applied in the direction of antibody medical ingredients, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., to achieve the effect of reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Link between PEG-poly (AGE-Suc) and OVA
[0083] Dissolve 1mg OVA (purchased from Sigma) in 2ml PBS containing 25mmol / L DTT (dithiothreitol), stir at room temperature for two hours, and remove unreacted DTT with PD-10 desalting cartridge (purchased from GE Healthcare) Small molecular substances, to obtain OVA with broken disulfide bonds, freeze-dried for use. Dissolve PEG-poly(AGE-Suc) and the linking agent SPDP (purchased from Pierce Biotechnology) PEG-poly(AGE-Suc): SPDP=1:1.2 in 1.5ml PBS-EDTA buffer (composition 100 mmol / LNa 3 PO 4 , 150 mmol / L NaCl, 1 mmol / L EDTA, pH=7.5), stir at room temperature for 45 minutes, and use a PD-10 desalting cartridge to remove unreacted SPDP. Dissolve the broken disulfide bond OVA in 1ml PBS-EDTA, and then add it to the above desalted PEG-poly(AGE-Suc) solution (molar ratio PEG-poly(AGE-Suc):OVA=1.2:1) , Stir overnight. Use double-distilled water as the mobile phase, pass through a PD-10 desalting cartridge to remove small molecules ...
Embodiment 2
[0086] PEG-poly (AGE-Suc) and FITC (fluorescein isothiocyanate) link
[0087] Dissolve PEG-poly(AGE-Suc) and FITC (purchased from Sigma) in 10ml Na at a molar ratio of 1:1.8 2 CO 3 -NaHCO 3 In the buffer solution (pH=9.6), keep in the dark and stir for 16h, dialyze with a 1000Da dialysis bag in the dark for 3 days, and freeze-dry.
[0088]
Embodiment 3
[0090] Preparation of aluminum hydroxide-OVA-CpG nanoparticles
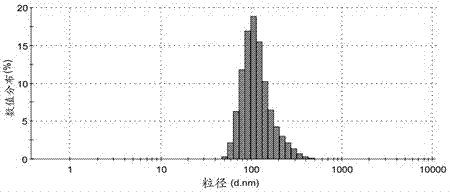
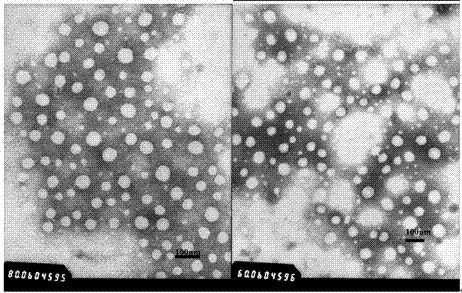
[0091] To 370μl 100mmol / L pH=7.6 HEPES buffer (37μmol), add 185μl 5mg / ml PEG-poly(AGE-Suc)-OVA solution (Example 1, the same below) (0.925mg), 10μl 1782μg / ml CpG- ODN (purchased from Shanghai Shenggong) (17.82μg), stir and mix well; draw 555μl 1.67mmol / L Al 2 (SO 4 ) 3 The solution (0.927μmol) was added to the above mixed solution and stirred for 30s to obtain aluminum hydroxide-OVA-CpG nanoparticles. Use Malvern Nano-ZS 90 particle size analyzer to detect particle size, see the results figure 2 . Take a small amount of sample and test with transmission electron microscope, see the results image 3 .
[0092]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com