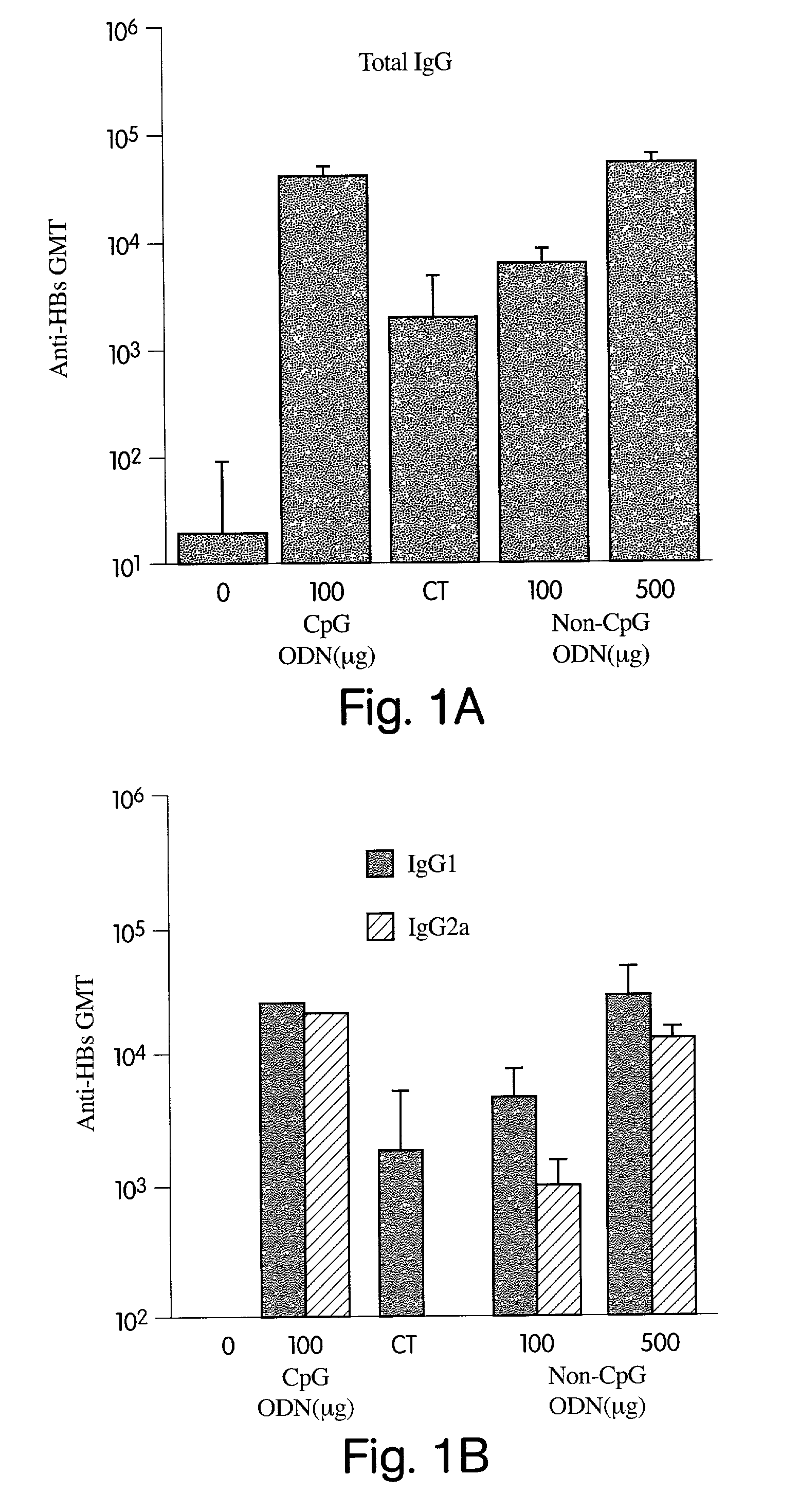
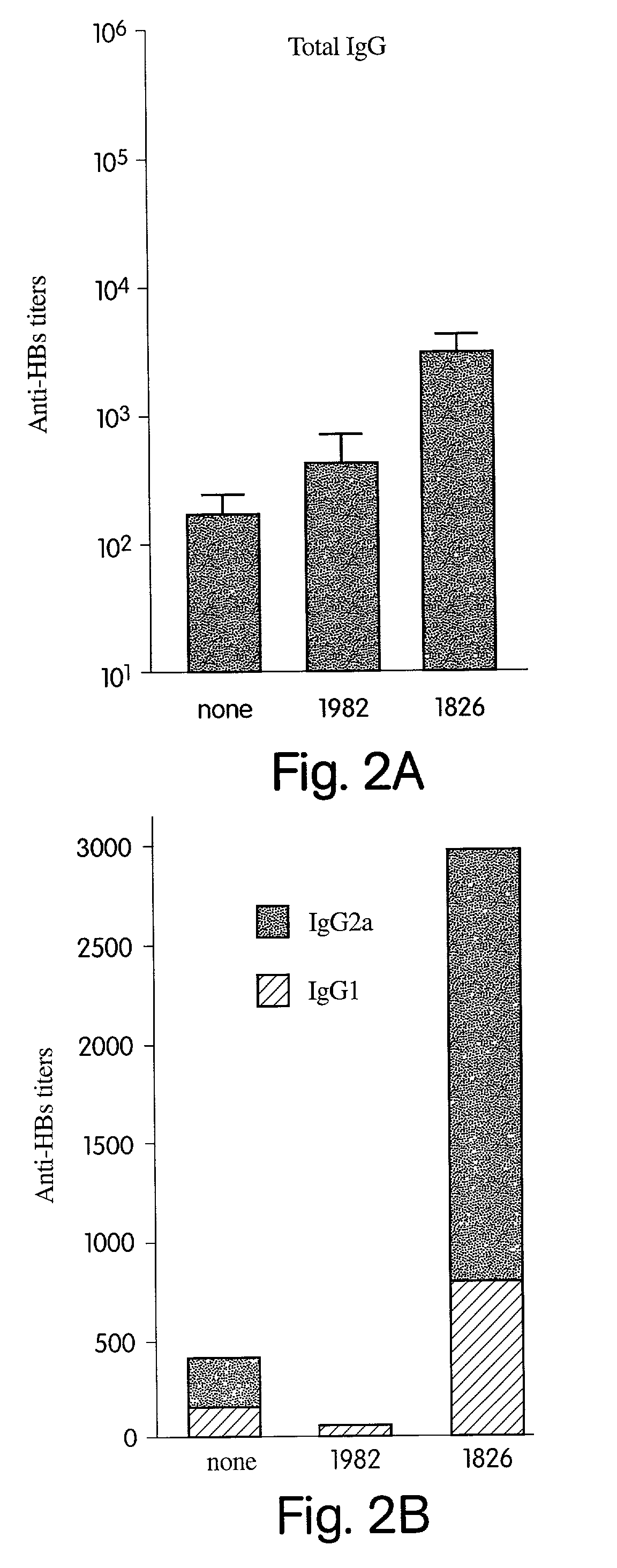
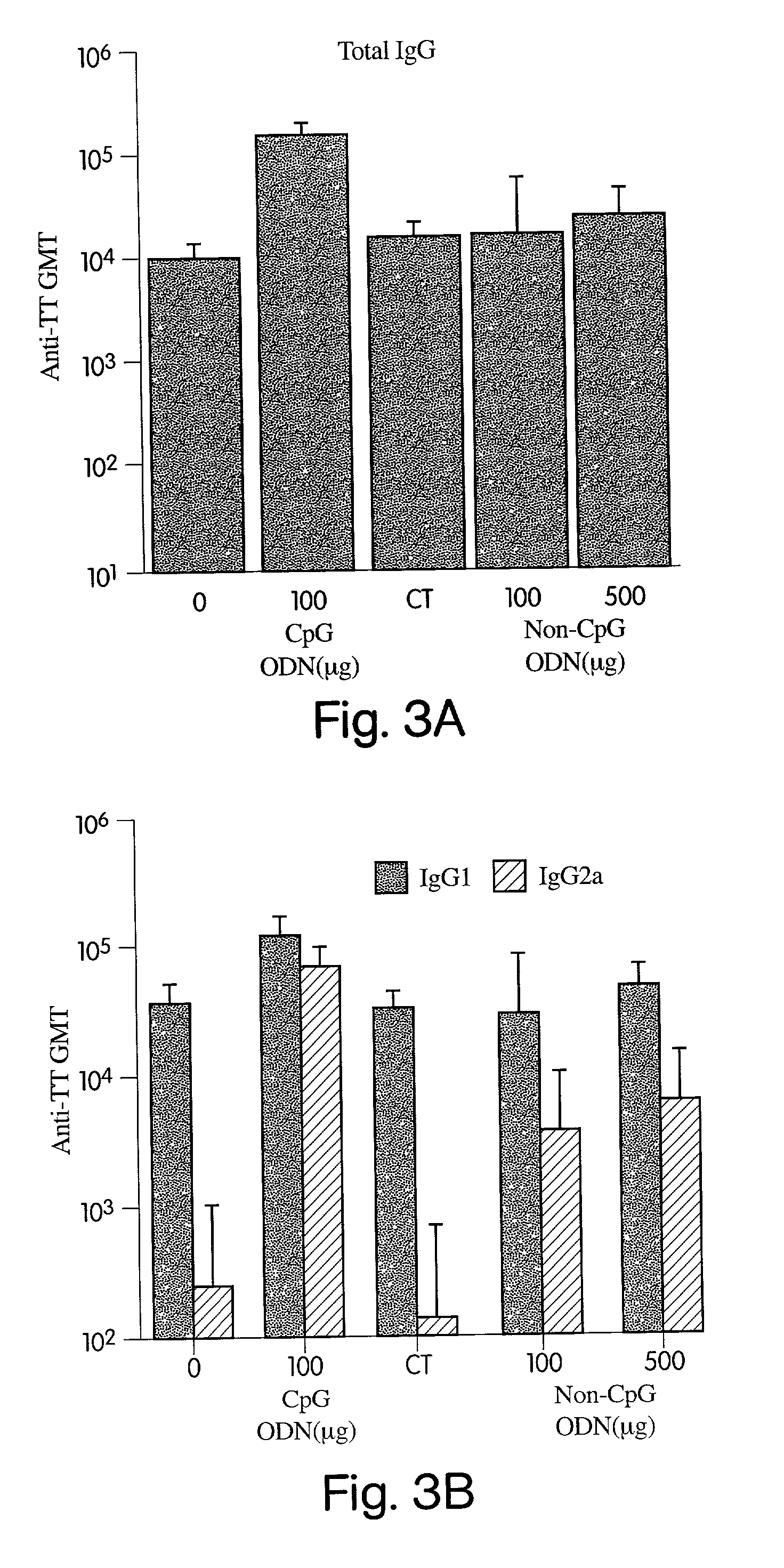
Patents
Literature
31 results about "Th1 immune response" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Immunostimulatory nucleic acids for inducing a Th2 immune response
Owner:OTTAVA HEALTH RES INST (CA)
Immunogenic compositions for Chlamydia trachomatis
PendingUS20060034871A1Enhance immune responseAntibacterial agentsBacterial antigen ingredientsDiseaseAdjuvant
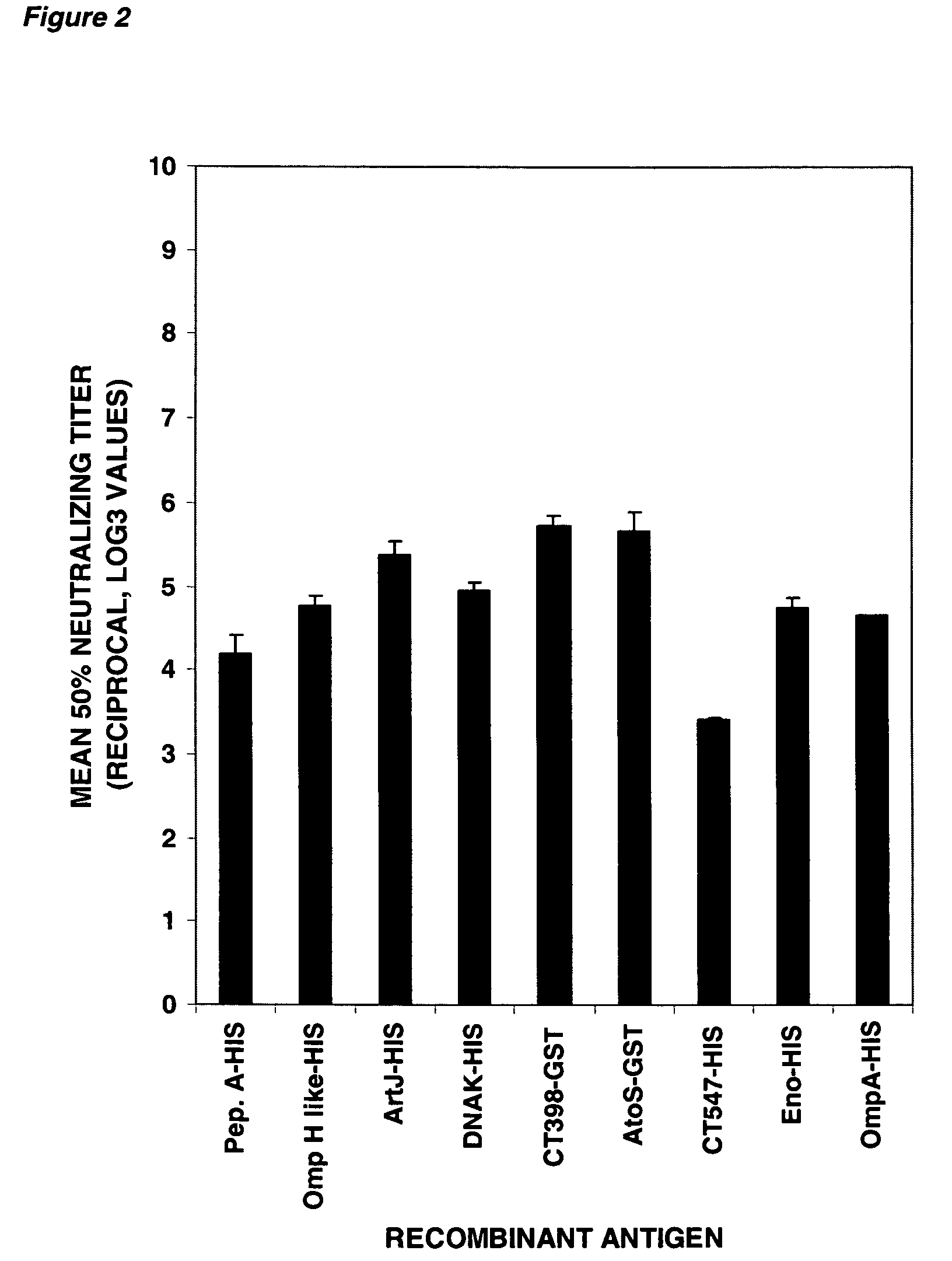
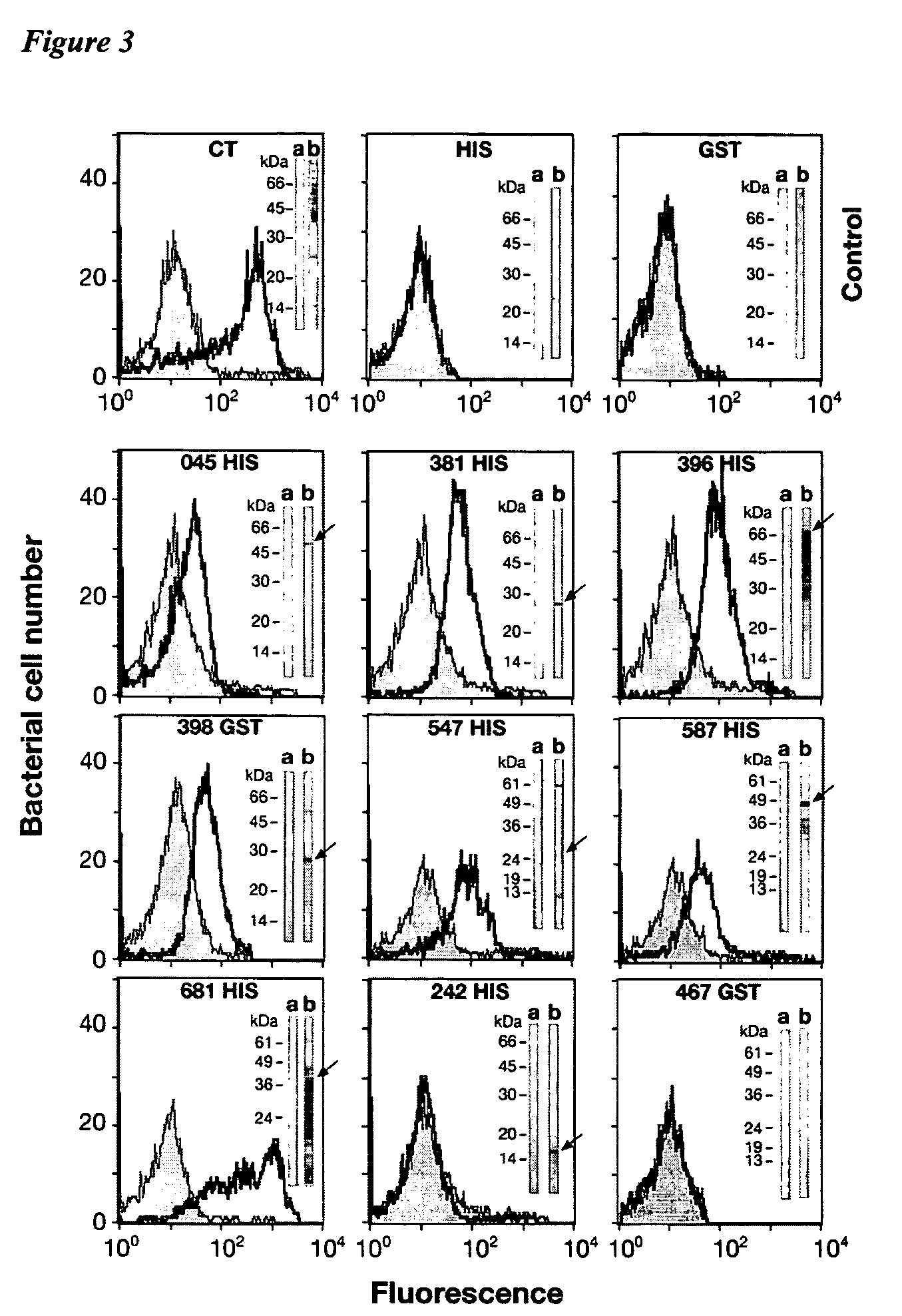
The invention relates to immunogenic compositions comprising combinations of Chlamydia trachomatis antigens and their use in vaccines. The composition may comprise at least two components, one component of which comprises Chlamydia trachomatis antigens for eliciting a Chlamydia trachomatis specific TH1 immune response and another component of which comprises antigens for eliciting a Chlamydia trachomatis specific TH2 immune response. The invention further relates to an immunogenic composition comprising a Chlamydia trachomatis Type III secretion system (TTSS) regulatory protein and a Chlamydia trachomatis Type III secretion system (TTSS) secreted protein or a fragment thereof. The invention further relates to the use of combinations of adjuvants for use with antigens associated with a sexually transmissible disease, such as Chlamydia trachomatis antigens. Preferred adjuvant combinations include mineral salts, such as aluminium salts and oligonucleotides comprising a CpG motif. The invention further provides a combination of Chlamydia trachomatis antigens comprising a Chlamydia trachomatis antigen that is conserved over at least two serovars.
Owner:NOVARTIS AG
Microemulsions with adsorbed macromolecules and microparticles
InactiveUS8206749B1Powerful toolStimulate immune responseAntibacterial agentsDigestive systemHydroxybutyric acidAdjuvant
Microparticles with adsorbent surfaces, methods of making such microparticles, and uses thereof, are disclosed. The microparticles comprise a polymer, such as a poly(α-hydroxy acid), a polyhydroxy butyric acid, a polycaprolactone, a polyorthoester, a polyanhydride, and the like, and are formed using cationic, anionic, or nonionic detergents. The surface of the microparticles efficiently adsorb biologically active macromolecules, such as DNA, polypeptides, antigens, and adjuvants. Also provided are compositions of an oil droplet emulsion having a metabolizable oil and an emulsifying agent. Immunogenic compositions having an immunostimulating amount of an antigenic substance, and an immunostimulating amount of an adjuvant composition are also provided. Methods of stimulating an immune response, methods of immunizing a host animal against a viral, bacterial, or parasitic infection, and methods of increasing a Th1 immune response in a host animal by administering to the animal an immunogenic composition of the microparticles, and / or microemulsions of the invention, are also provided.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
Microemulsions with adsorbed macromolecules and microparticles
InactiveUS20070116709A1Improve adsorption capacityStimulate immune responseAntibacterial agentsPowder deliveryPolyesterAdjuvant
Microparticles with adsorbent surfaces, methods of making such microparticles, and uses thereof, are disclosed. The microparticles comprise a polymer, such as a poly(α-hydroxy acid), a polyhydroxy butyric acid, a polycaprolactone, a polyorthoester, a polyanhydride, and the like, and are formed using cationic, anionic, or nonionic detergents. The surface of the microparticles efficiently adsorb biologically active macromolecules, such as DNA, polypeptides, antigens, and adjuvants. Also provided are compositions of an oil droplet emulsion having a metabolizable oil and an emulsifying agent. Immunogenic compositions having an immunostimulating amount of an antigenic substance, and an immunostimulating amount of an adjuvant composition are also provided. Methods of stimulating an immune response, methods of immunizing a host animal against a viral, bacterial, or parasitic infection, and methods of increasing a Th1 immune response in a host animal by administering to the animal an immunogenic composition of the microparticles, and / or microemulsions of the invention, are also provided.
Owner:NOVARTIS VACCINES & DIAGNOSTICS INC
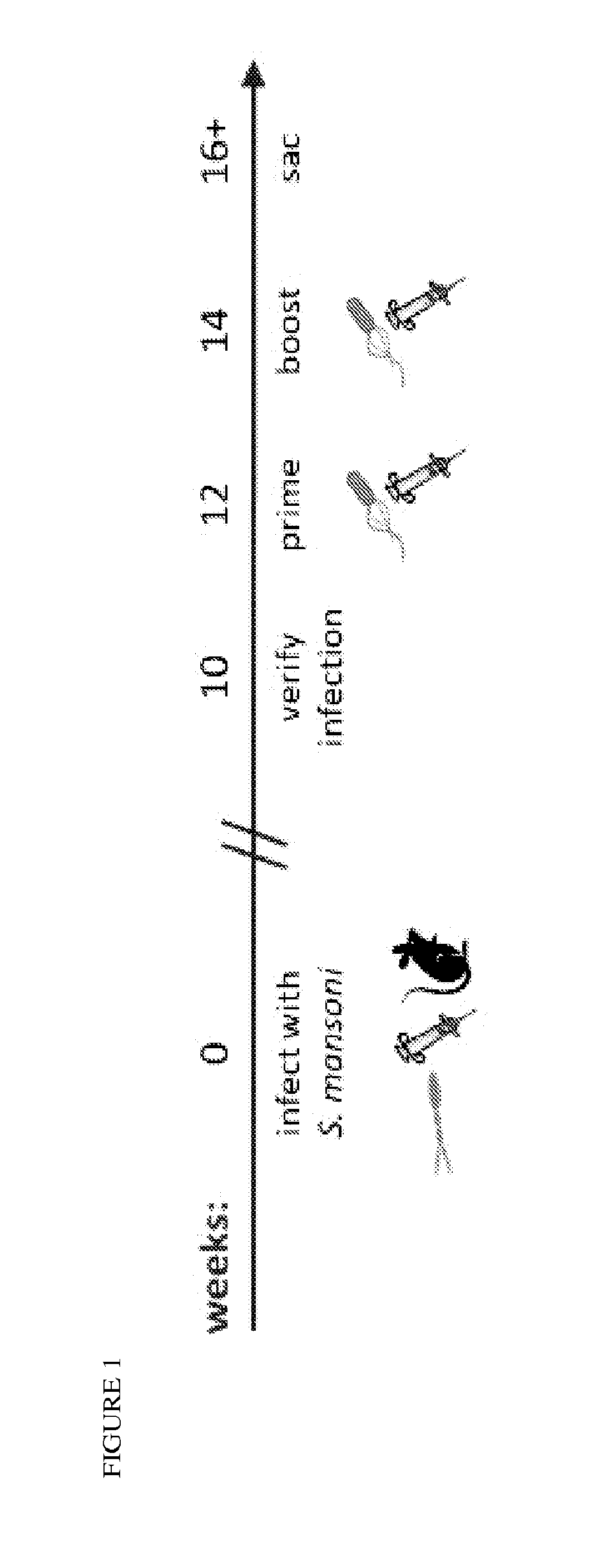
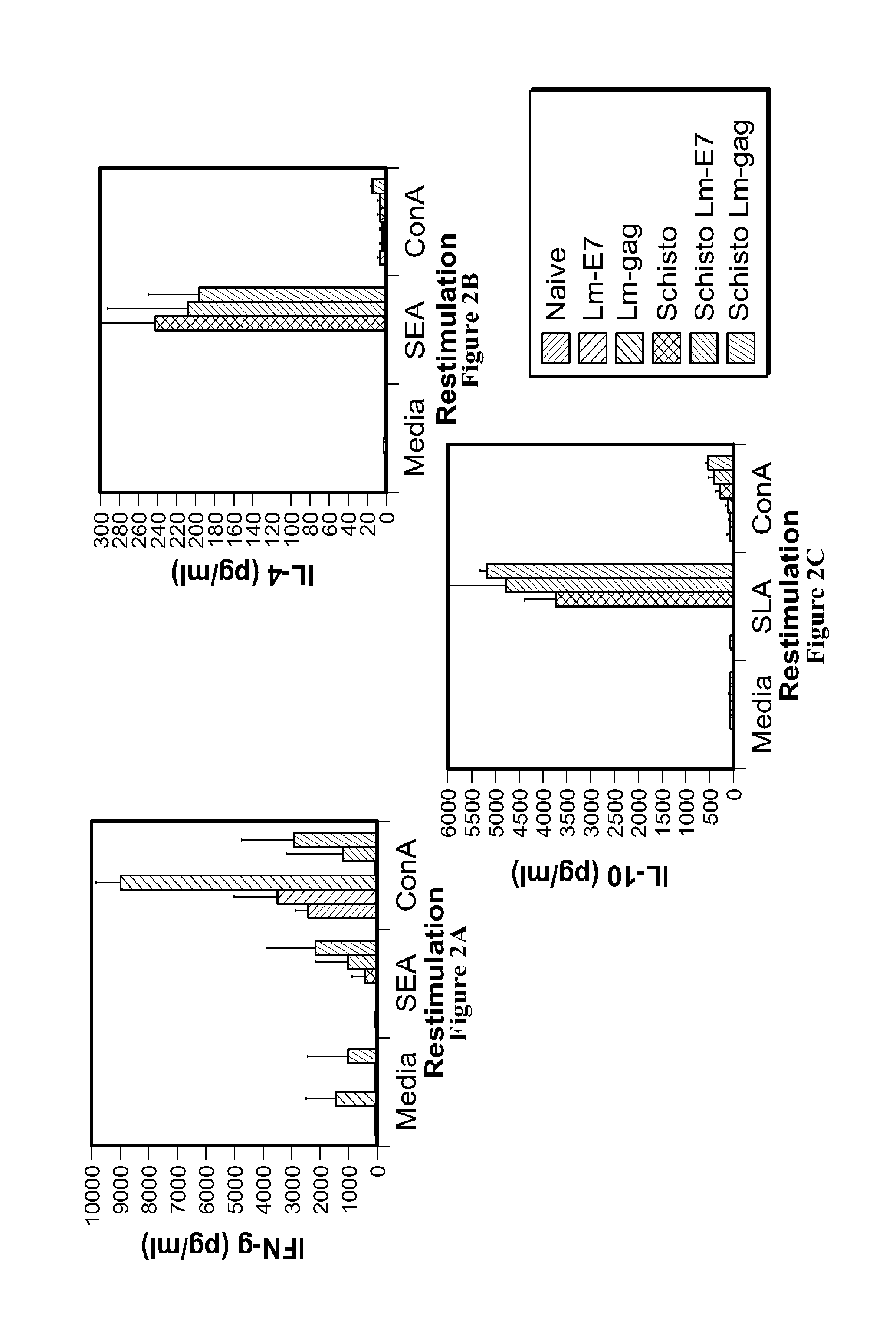
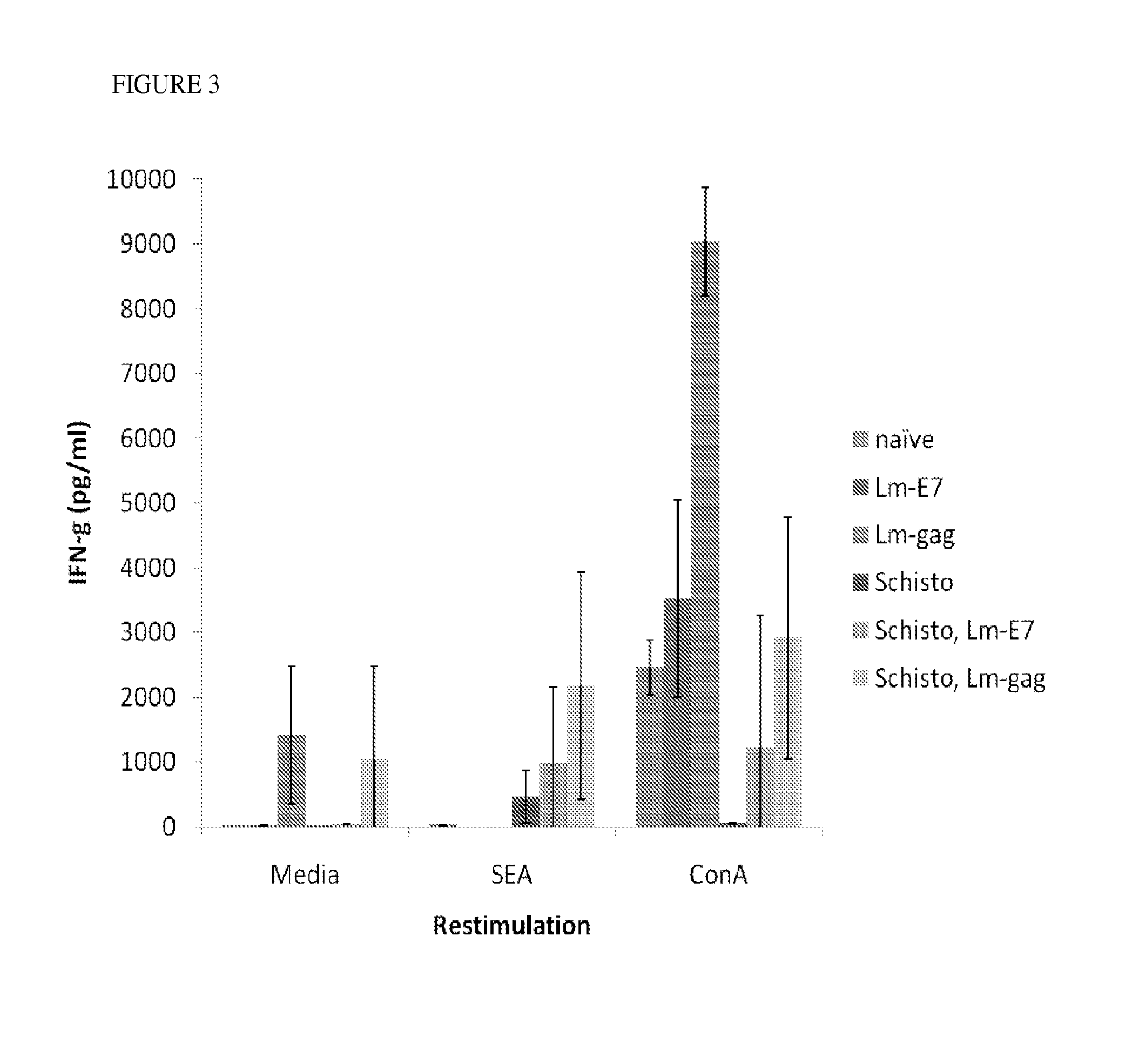
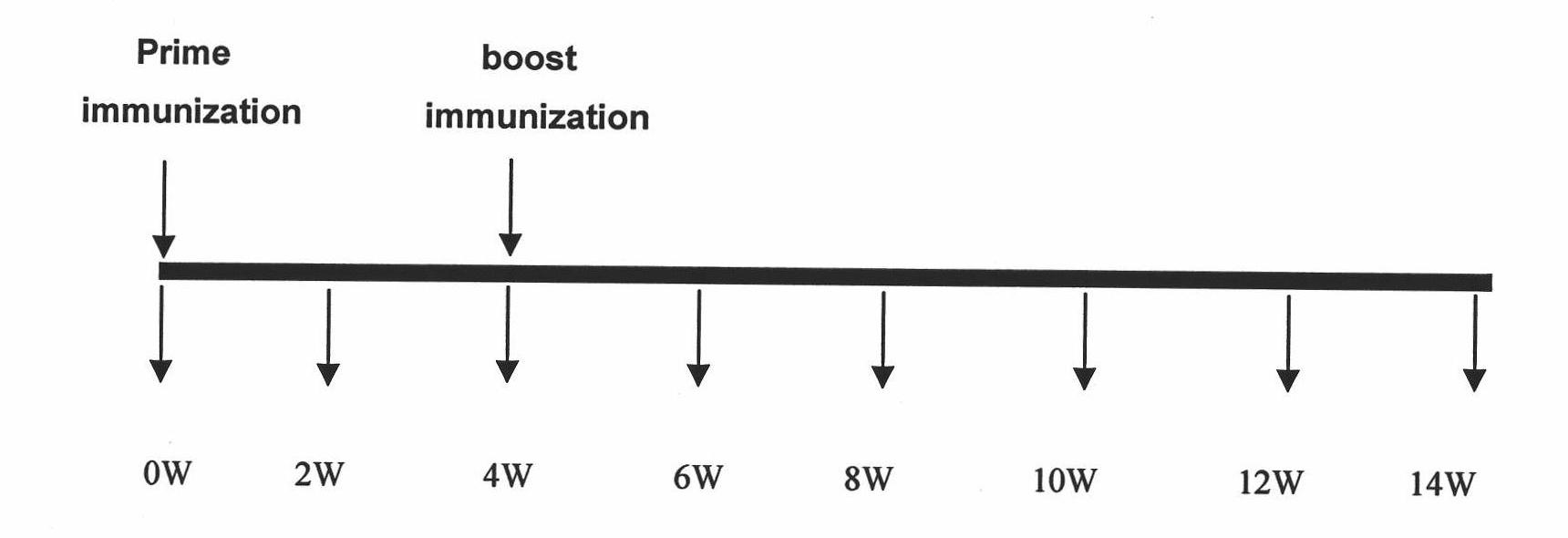
Use of listeria vaccine vectors to reverse vaccine unresponsiveness in parasitically infected individuals
This invention relates to methods of using a Listeria vaccine vector to induce a Th1 immune response in subjects having persistent Th2 immune response profiles.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Vaccine for controlling persistent infection of hepatitis B virus
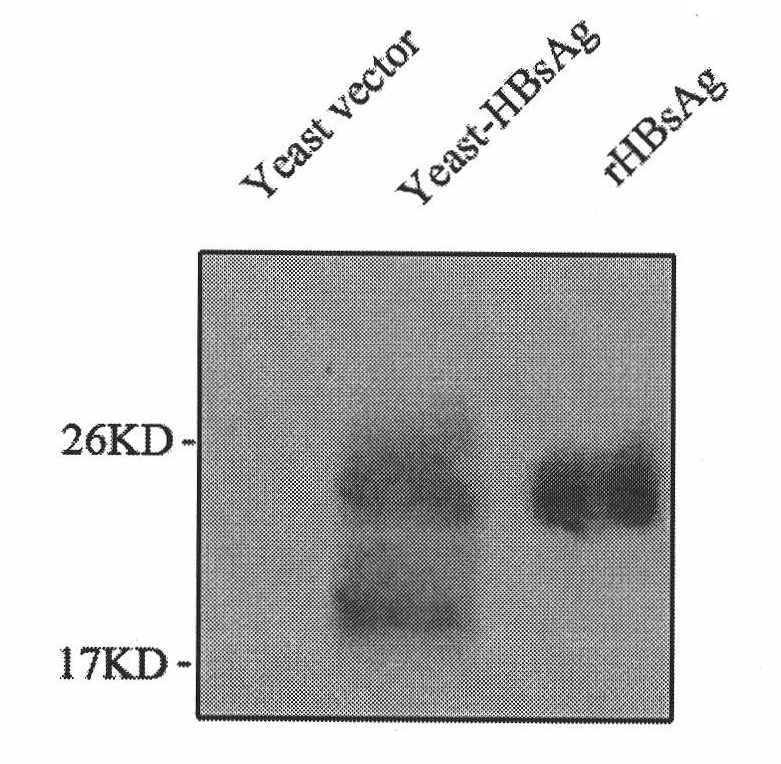
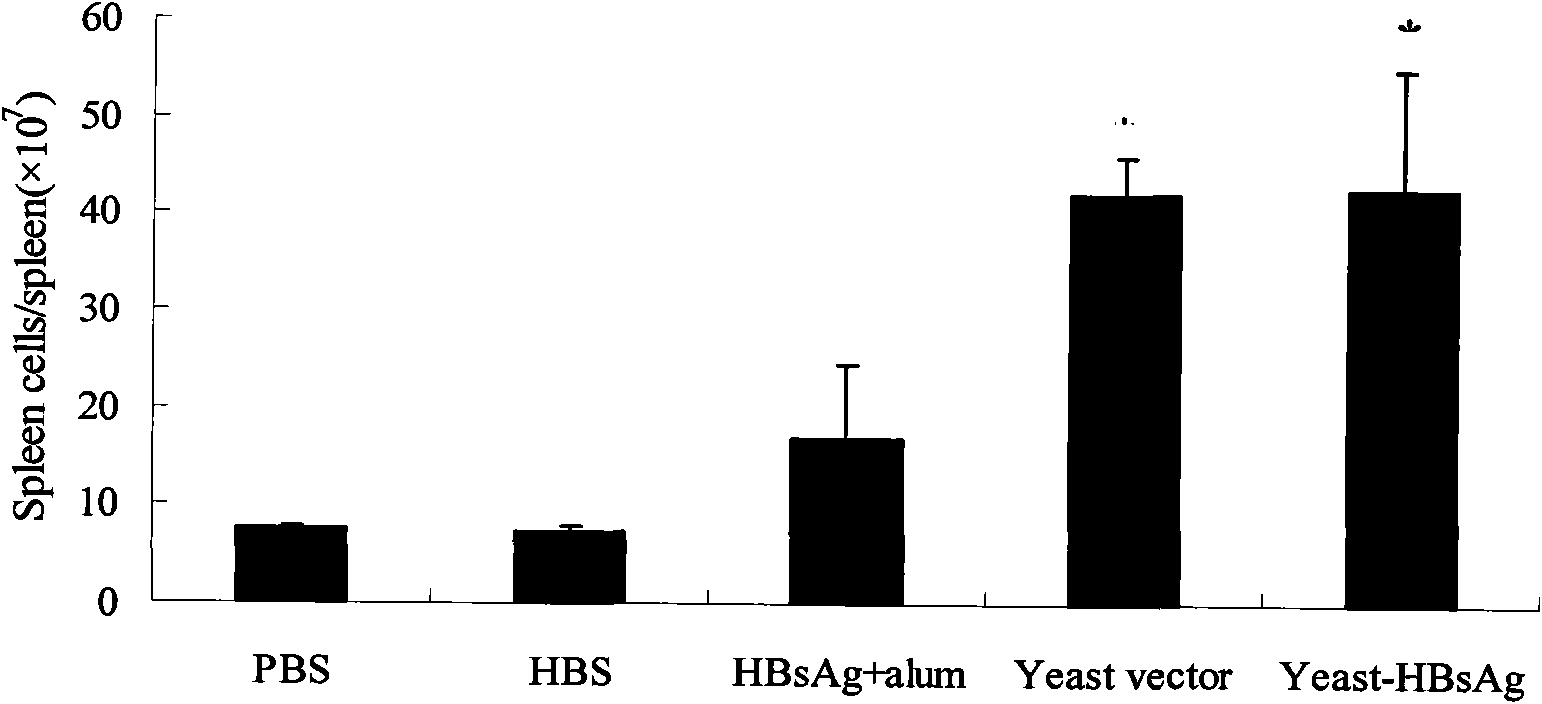
InactiveCN102038948AExcellent adjuvant effectPromote maturityAntiviralsAntibody medical ingredientsAdjuvantHepatitis B Surface Antigens
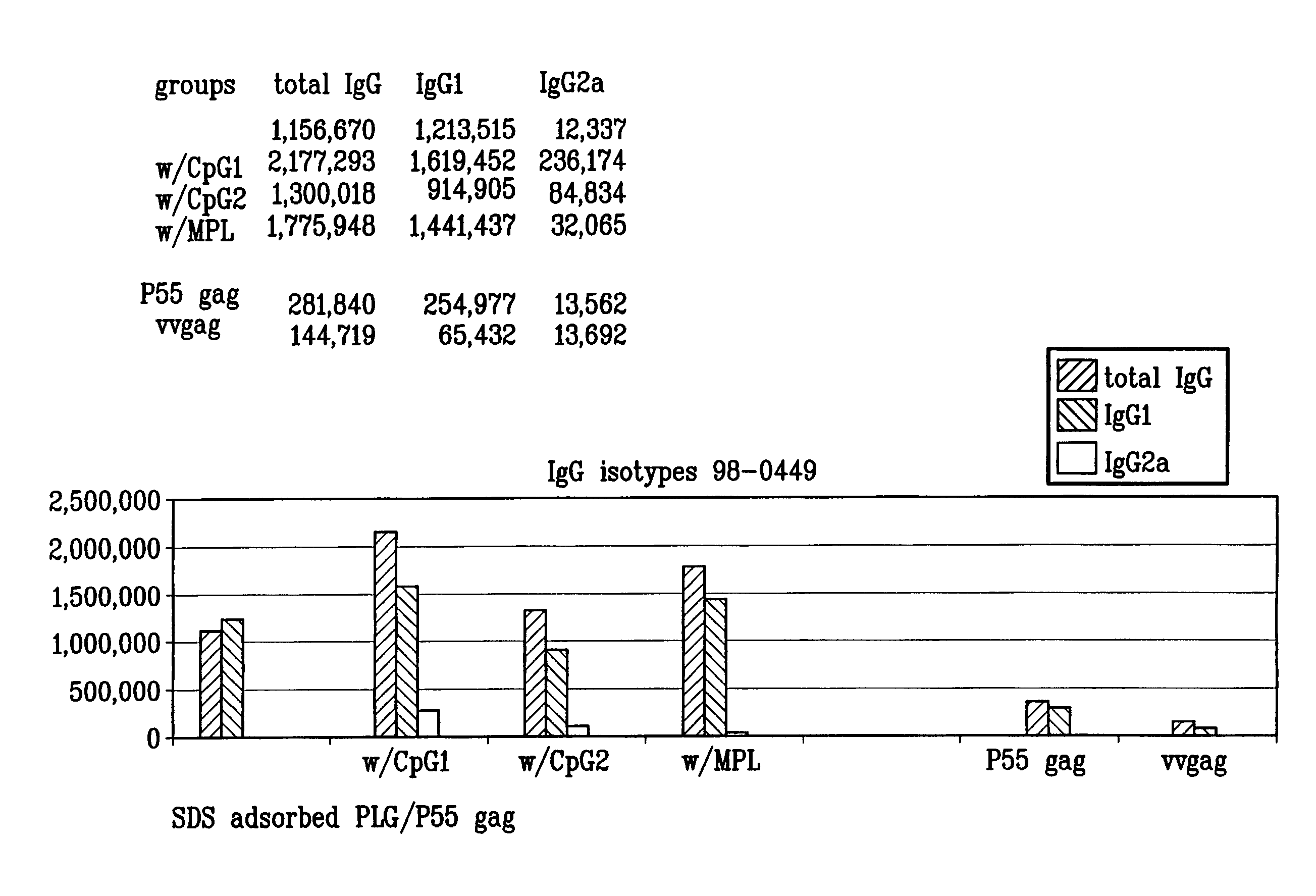
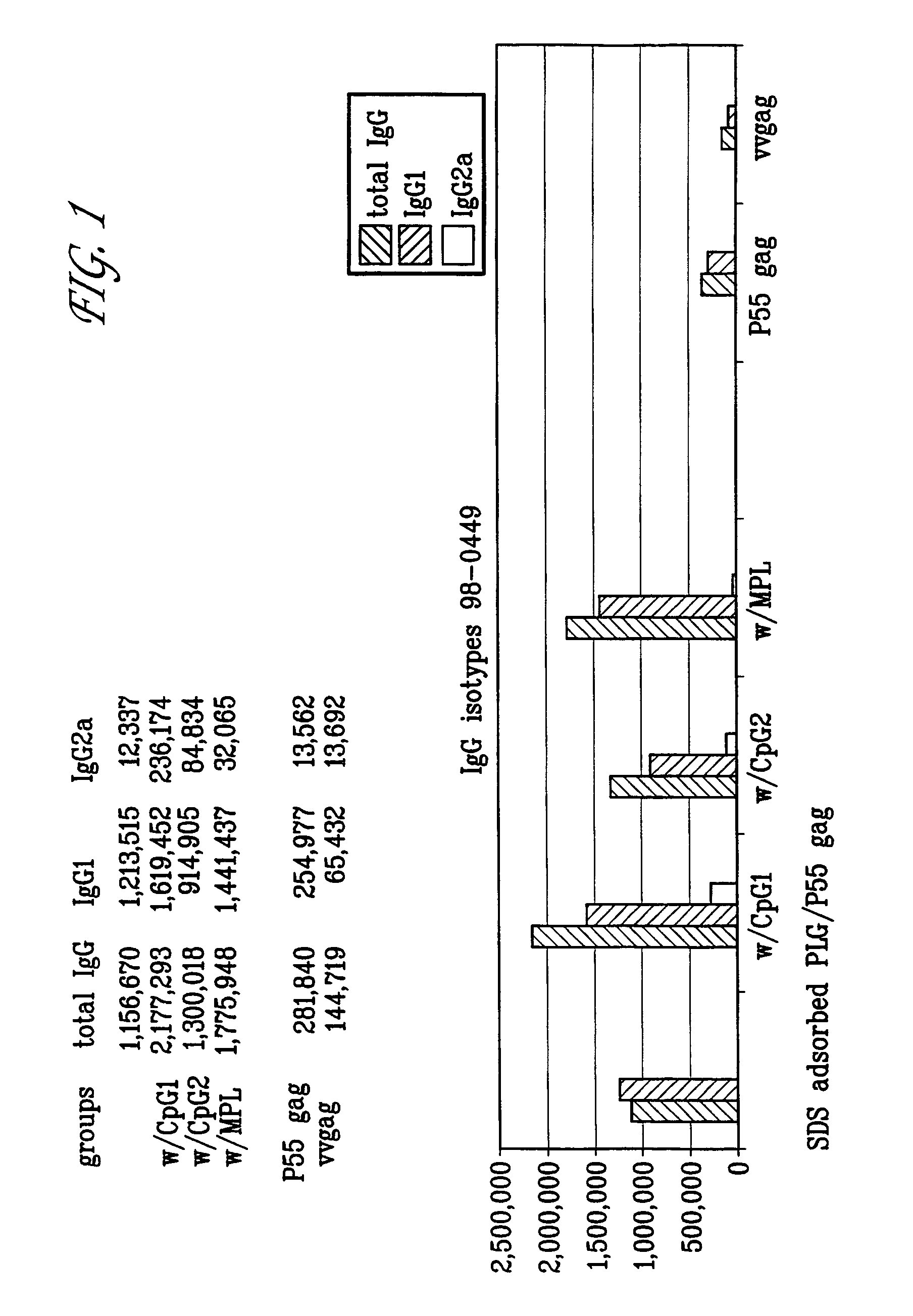
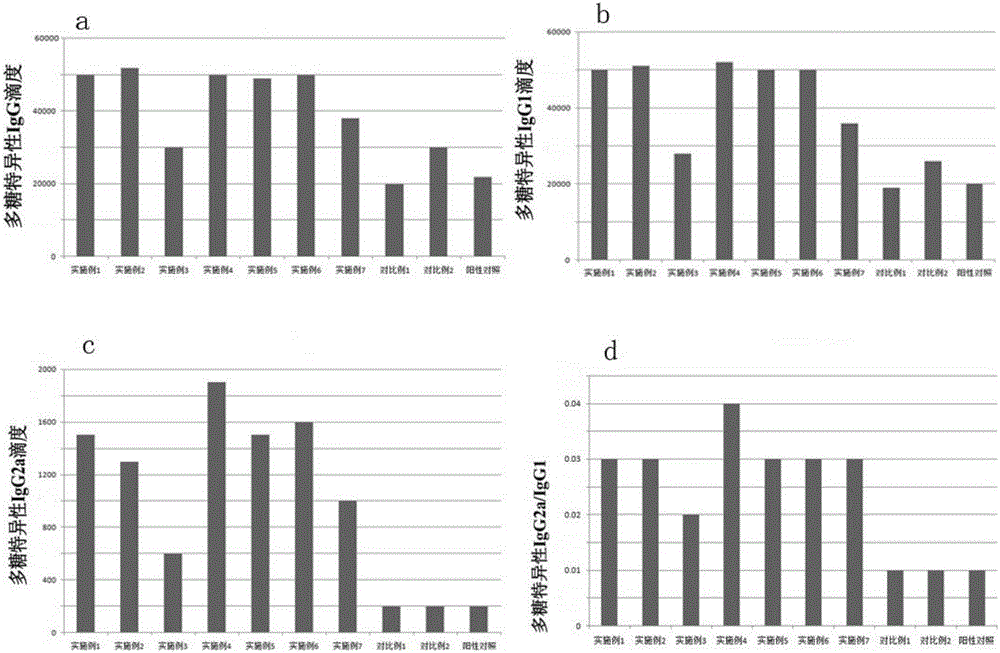
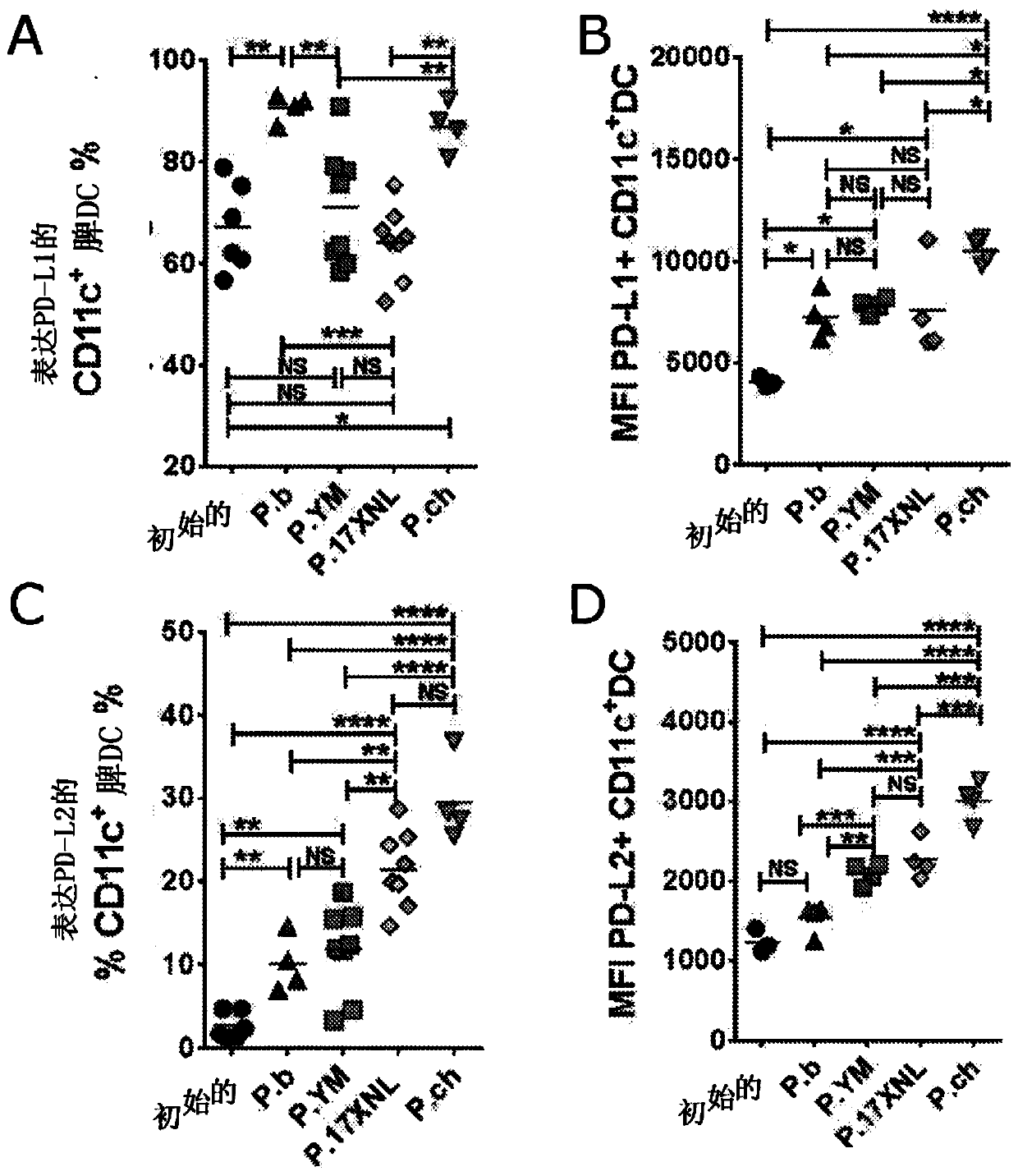
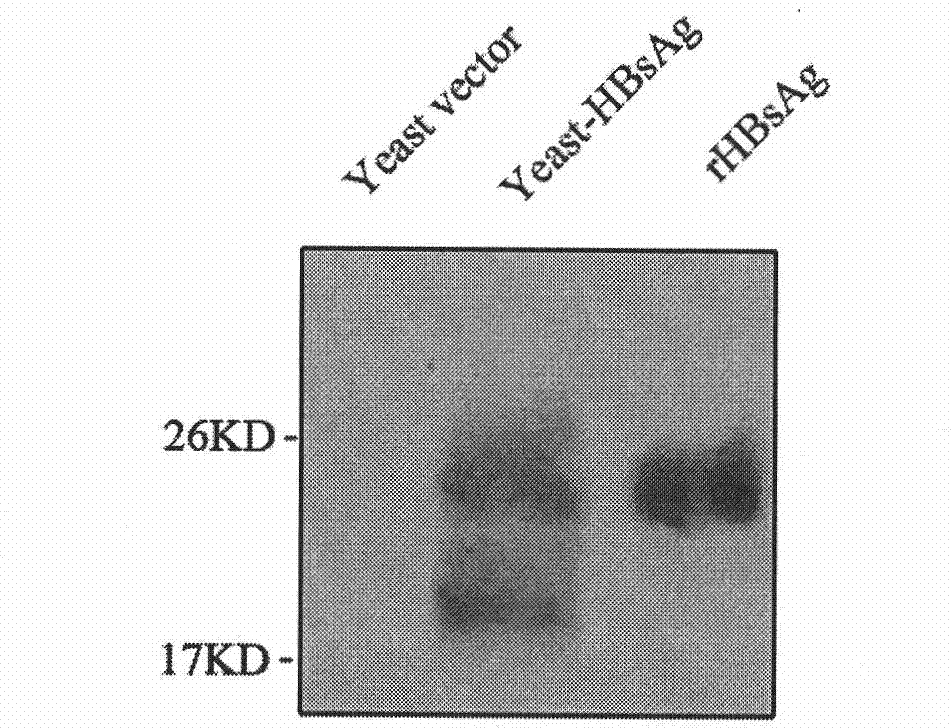
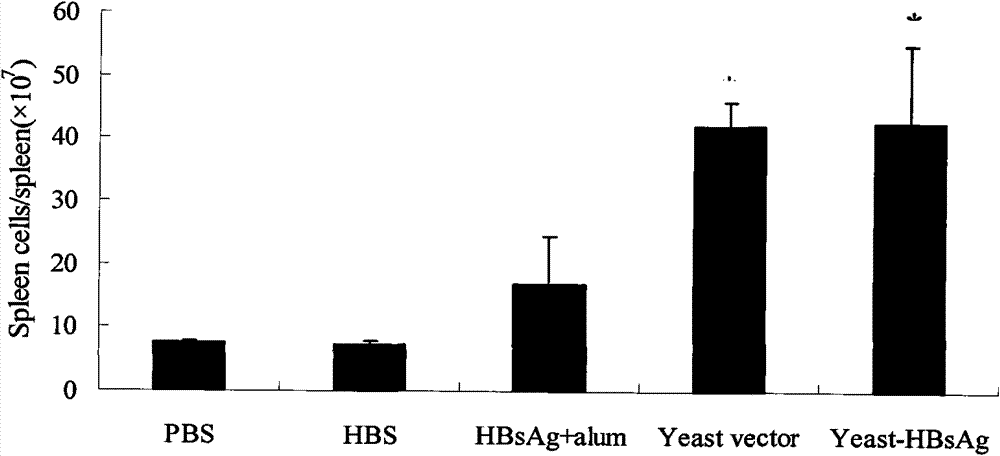
The invention belongs to the biotechnology field, and relates to a vaccine for controlling the persistent infection of hepatitis B virus. According to the invention, a Hansenula polymorpha cell that is heat inactivated and expresses hepatitis B surface antigen is adopted as the hepatitis B vaccine, wherein the HBsAg is an antigen part of the vaccine and the Hansenula polymorpha cell is an adjuvant part of the vaccine. Animal immunity experiment shows that: the vaccine can induce the aggregation of immune cells to the spleen, promote the maturation of DCs, and both induce Th1 immune response and enhance Th2 immune response in mice, including total IgG, IgG1, IgG2a, specific CTL activity, and the production ability of antigen-specific IFN-gama. The invention can make up for the deficiency of the impossibility of inducing Th1 immune response for traditional vaccines that take aluminium hydroxide as an adjuvant, and is helpful to the control of the persistent infection of hepatitis B virus.
Owner:FUDAN UNIV
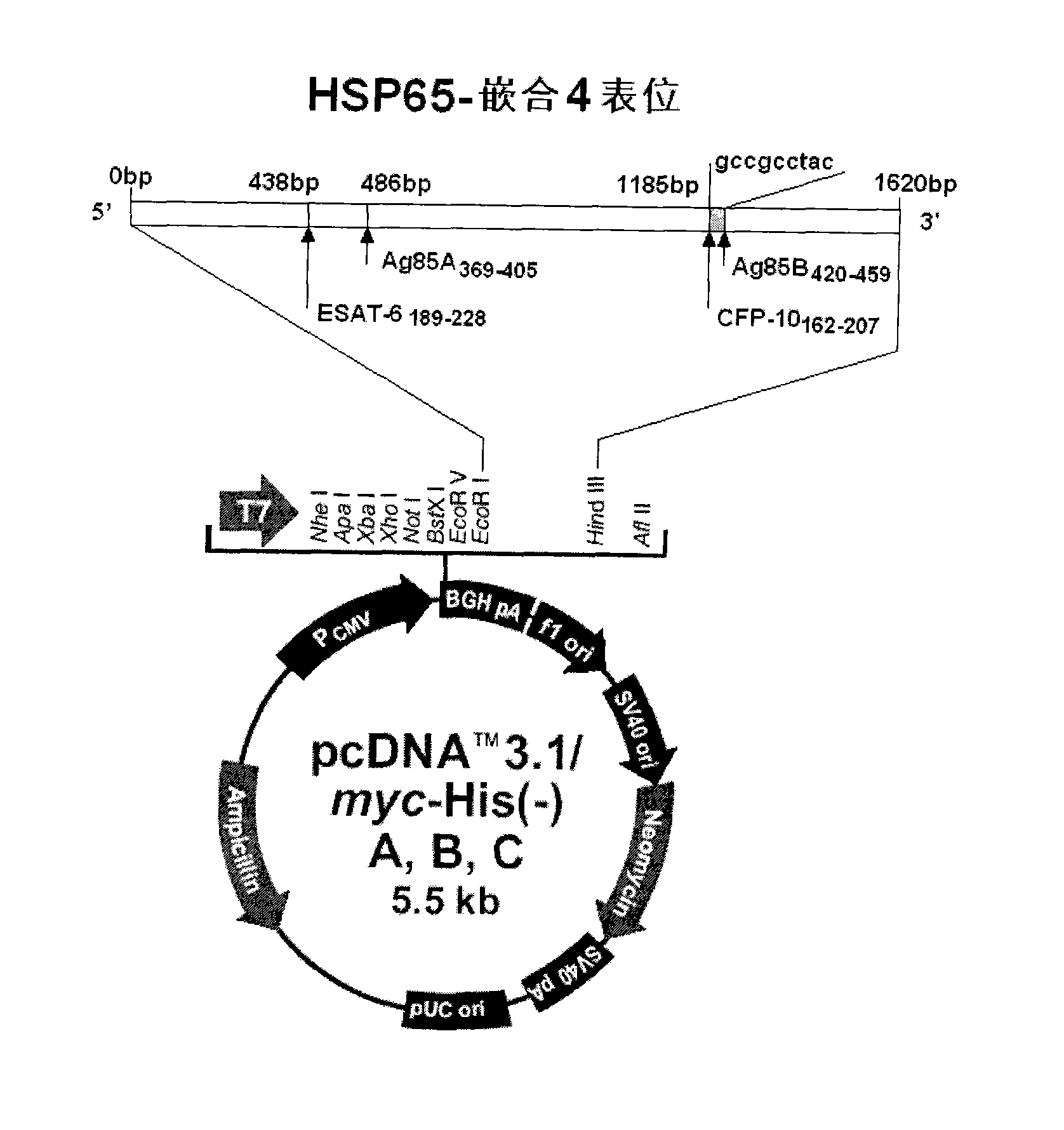
Tuberculosis gene vaccine based on T cell epitope as well as preparation method and use thereof
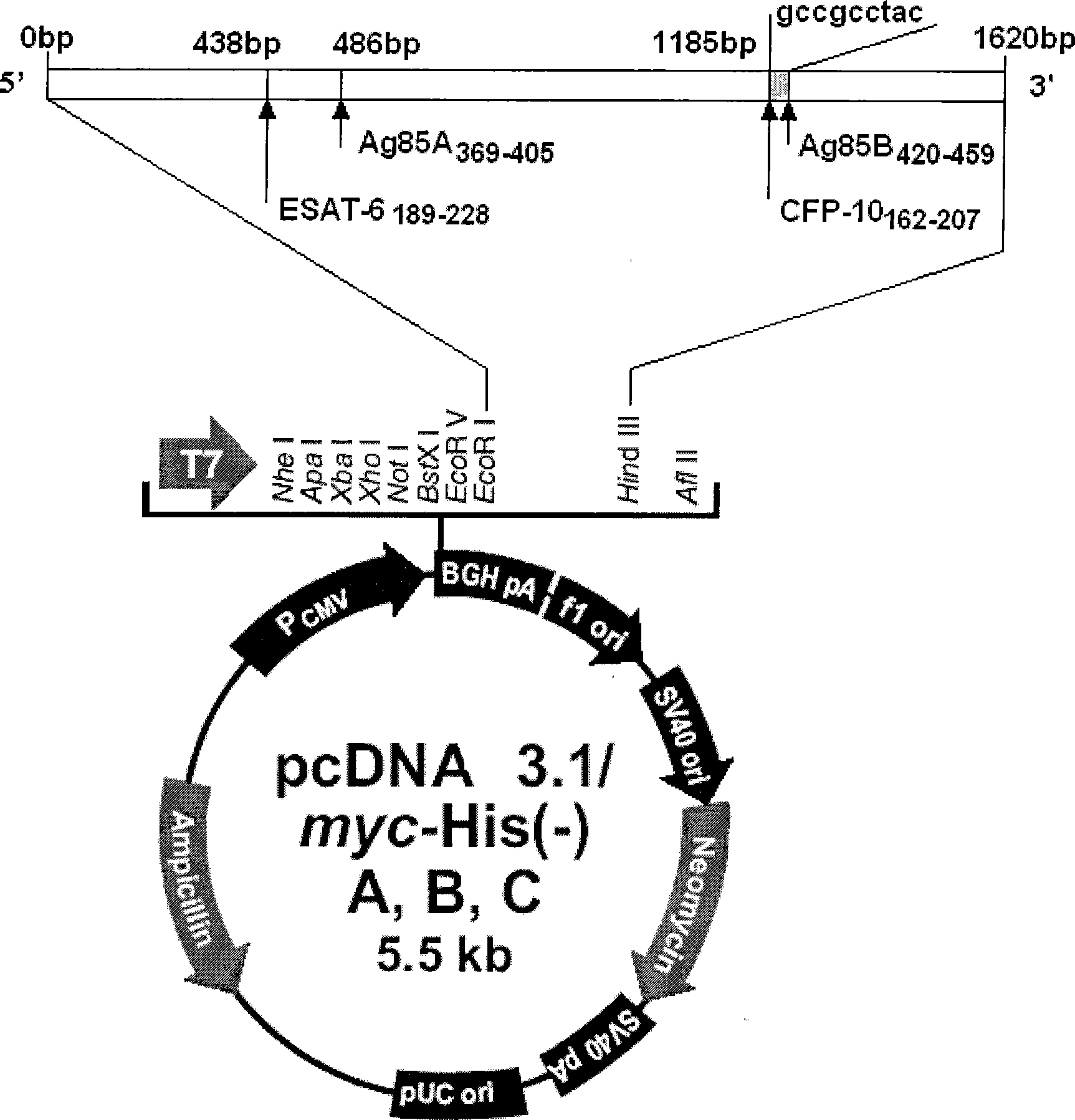
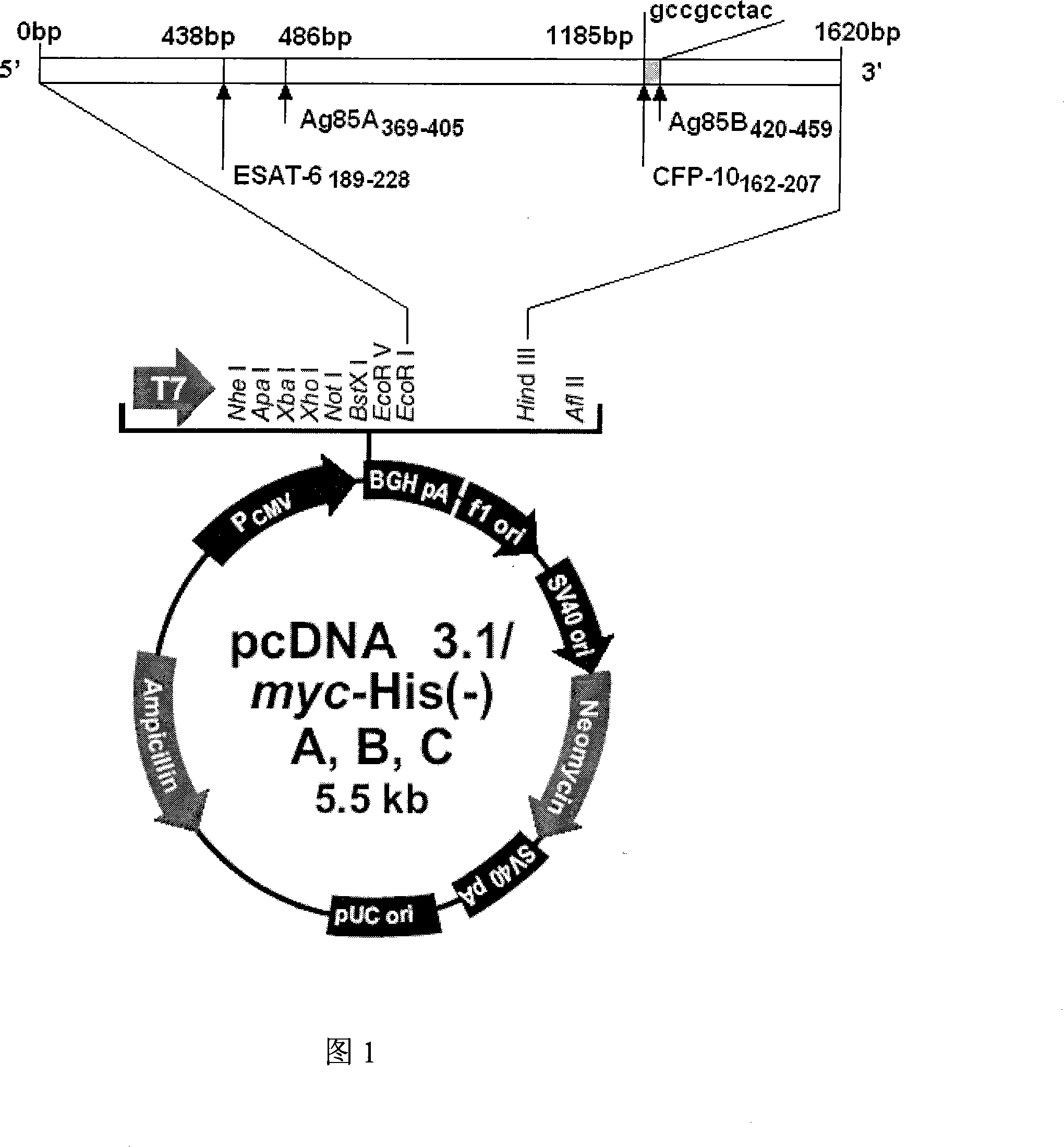
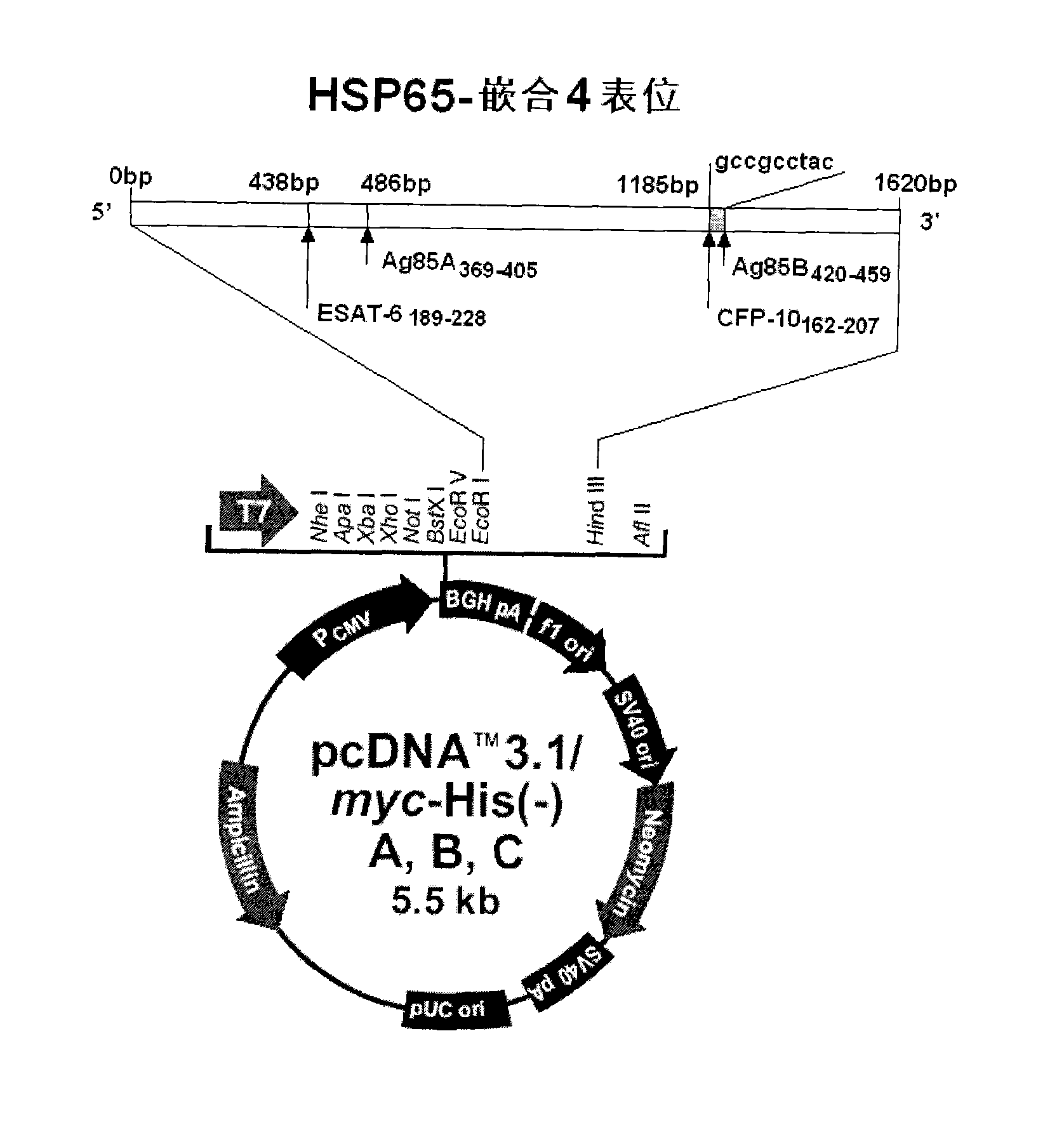
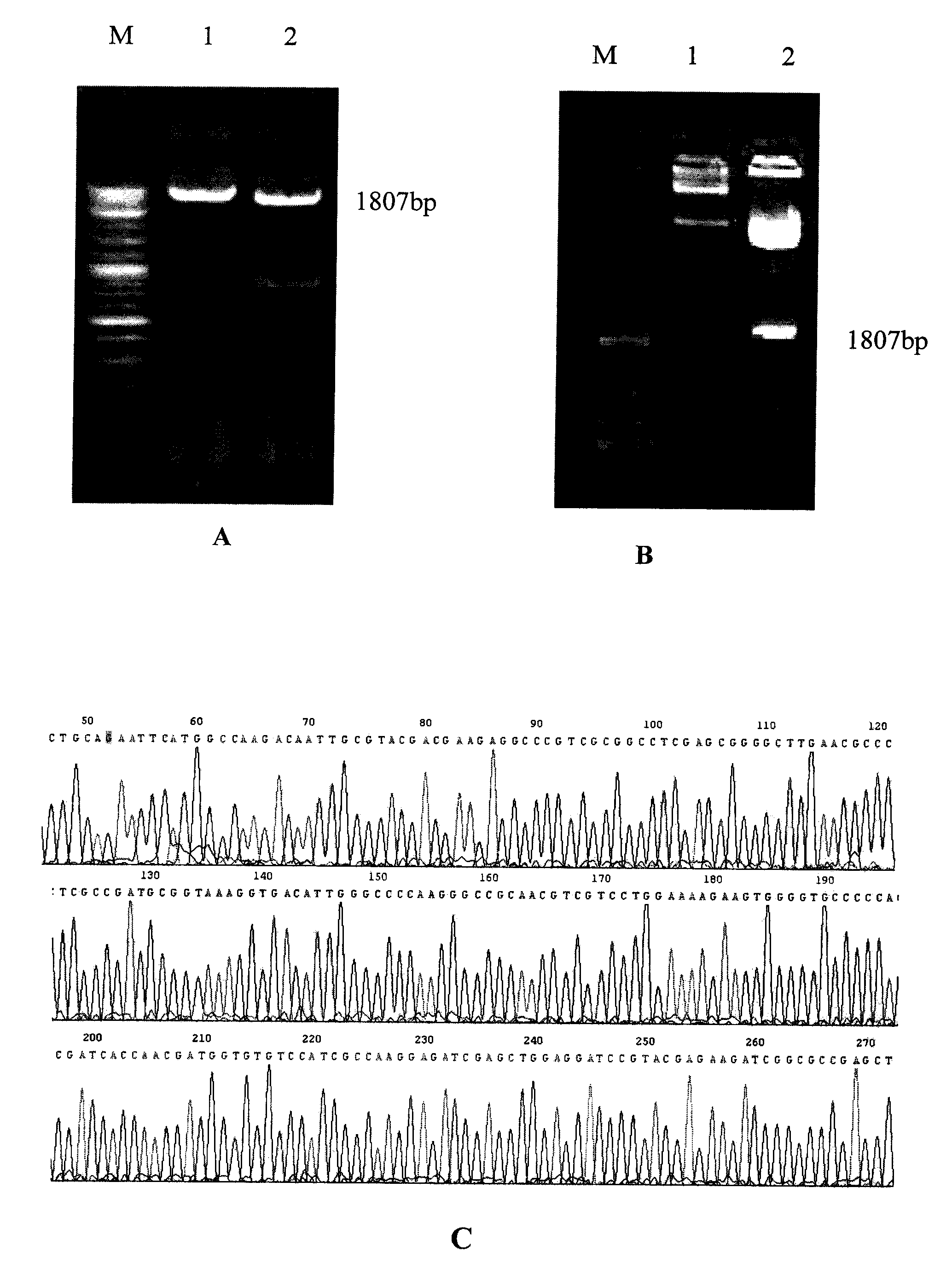
InactiveCN101451145AActivate immune responseDoes not affect the spatial structureAntibacterial agentsGenetic material ingredientsIntramuscular injectionTreating tuberculosis
The invention discloses a tuberculosis gene vaccine based on T cell epitopes, wherein a full-length gene, embedded with four T cell epitope polypeptide genes which come from mycobacterium tuberculosis antigen, of mycobacterium tuberculosis heat shock protein is inserted into a vector. The invention also discloses a method for preparing the vaccine, which comprises the following steps: four T cell epitope genes, namely EAST-6189-228, Ag85A369-405, CFP10162-207 and Ag85B420-459 which come from the mycobacterium tuberculosis antigen are inserted into an HSP65 full-length gene. The invention also discloses application of an ECANS tuberculosis gene vaccine. Through the intramuscular injection of the gene vaccine into an immune mouse, the experiment proves that the vaccine can induce a specific antibody which aims at a plurality of tuberculosis antigens to response, can induce stronger tuberculosis specific killing response, can induce Th1 immune response at the same time, secrete high-level IFN gamma, and is a good vaccine for preventing and treating tuberculosis.
Owner:FUDAN UNIV
Use of Listeria vaccine vectors to reverse vaccine unresponsiveness in parasitically infected individuals
This invention relates to methods of using a Listeria vaccine vector to induce a Th1 immune response in subjects having persistent Th2 immune response profiles.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA +1
Methods for Treating Allergic Disease
InactiveUS20110038882A1Safe to drinkSafe to eatPeptide/protein ingredientsAntipyreticT cellDisease cause
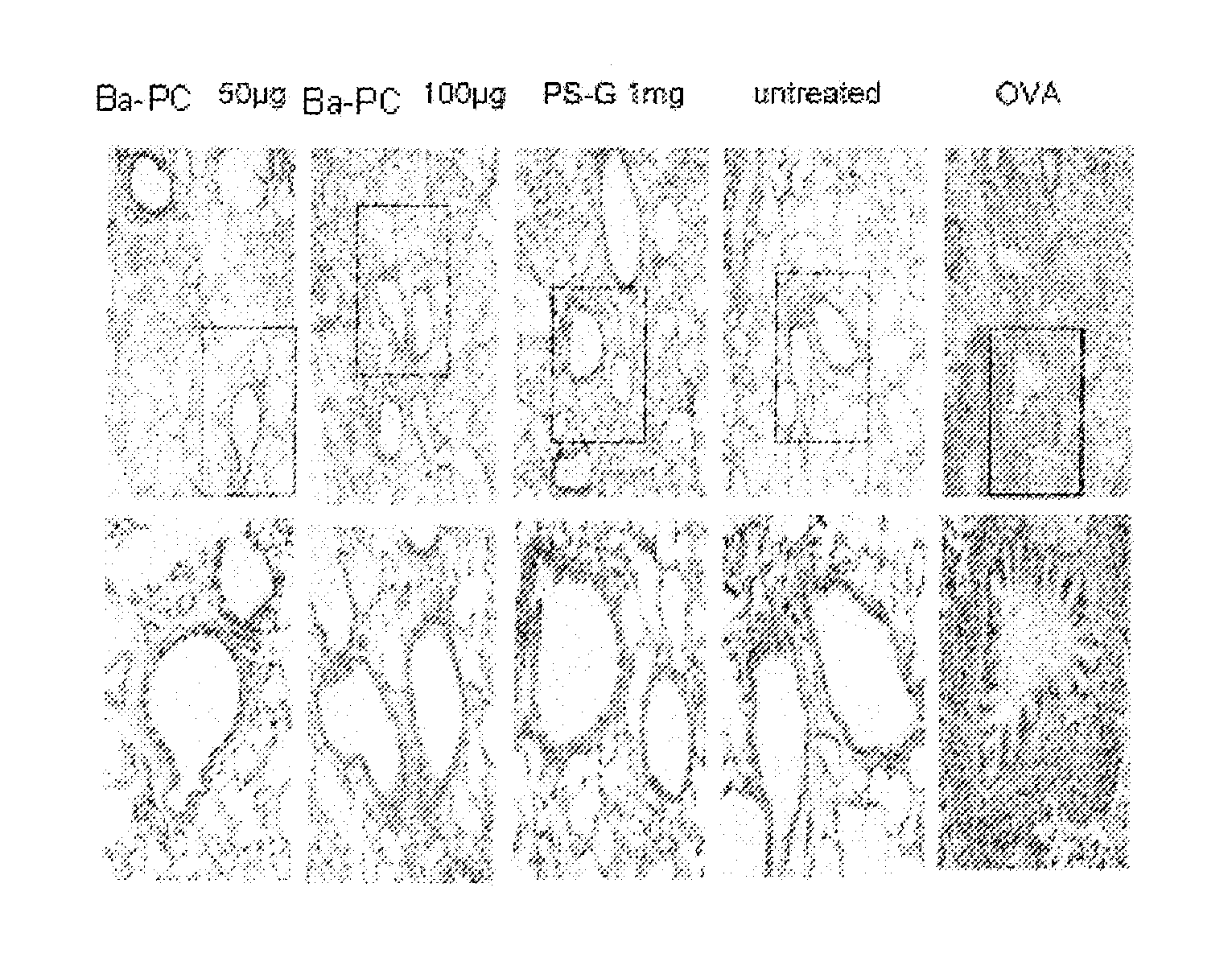
A method for treating or alleviating allergic disease in a mammal in need thereof having administering to the mammal a therapeutically effective amount of a pharmaceutical composition having phycocyanin is provided. A method for modulating balance between Th1 and Th2 immune response in a mammal in need thereof, having administering to the mammal an effective amount of phycocyanin, wherein immune response of the mammal is skewed toward the Th1 immune response is also provided. Phycocyanin from Bangia atropupurea (Ba-PC) is identified to regulate mammalian immunological response indicating that Ba-PC can direct skewed immune response toward Th1 response through modulating DC function, increase proliferative activity of antigen-specific T cells and alleviate airway inflammation, confirming that phycobiliproteins are effective in treating allergic disease.
Owner:NAT TAIWAN UNIV
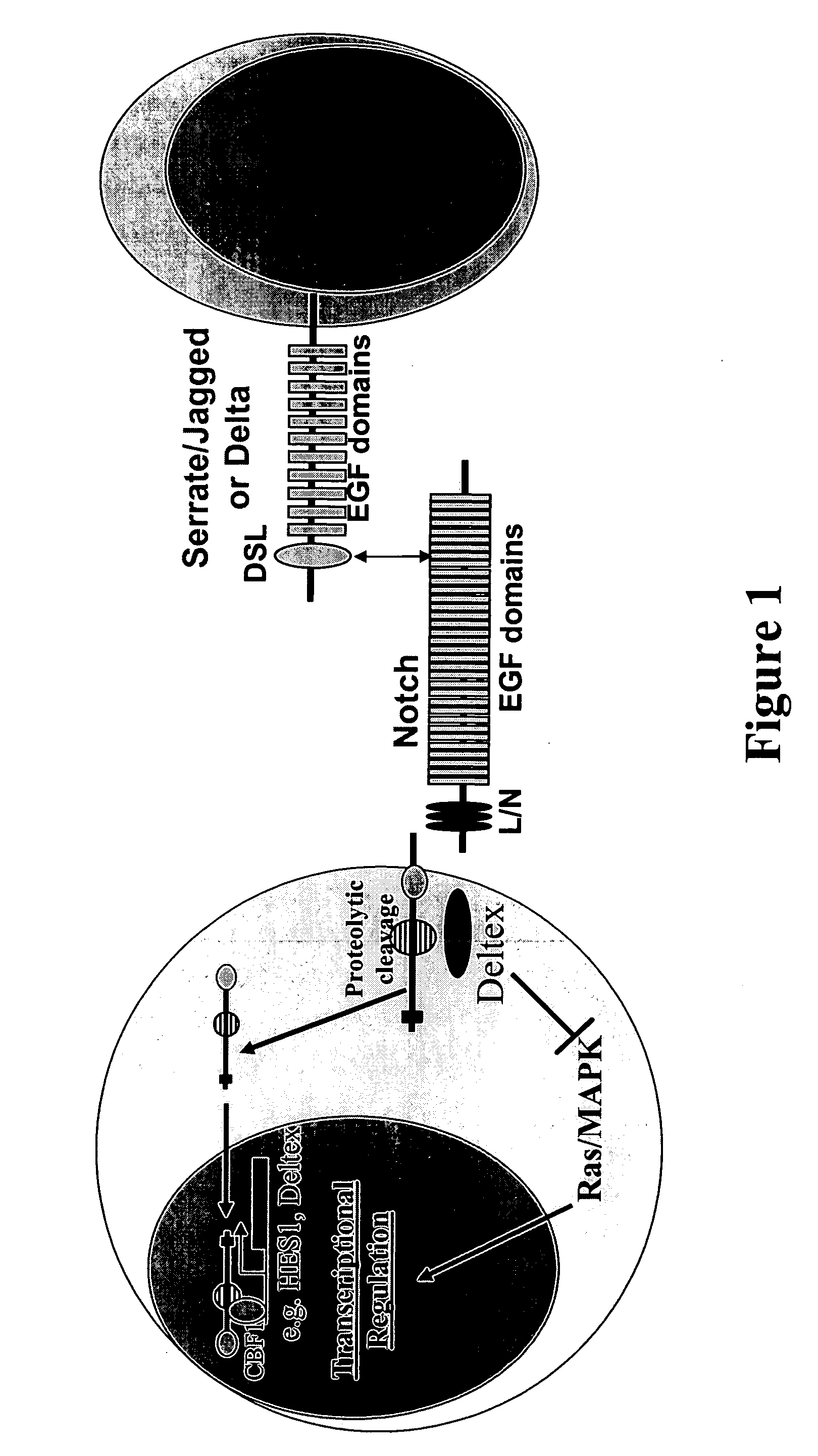
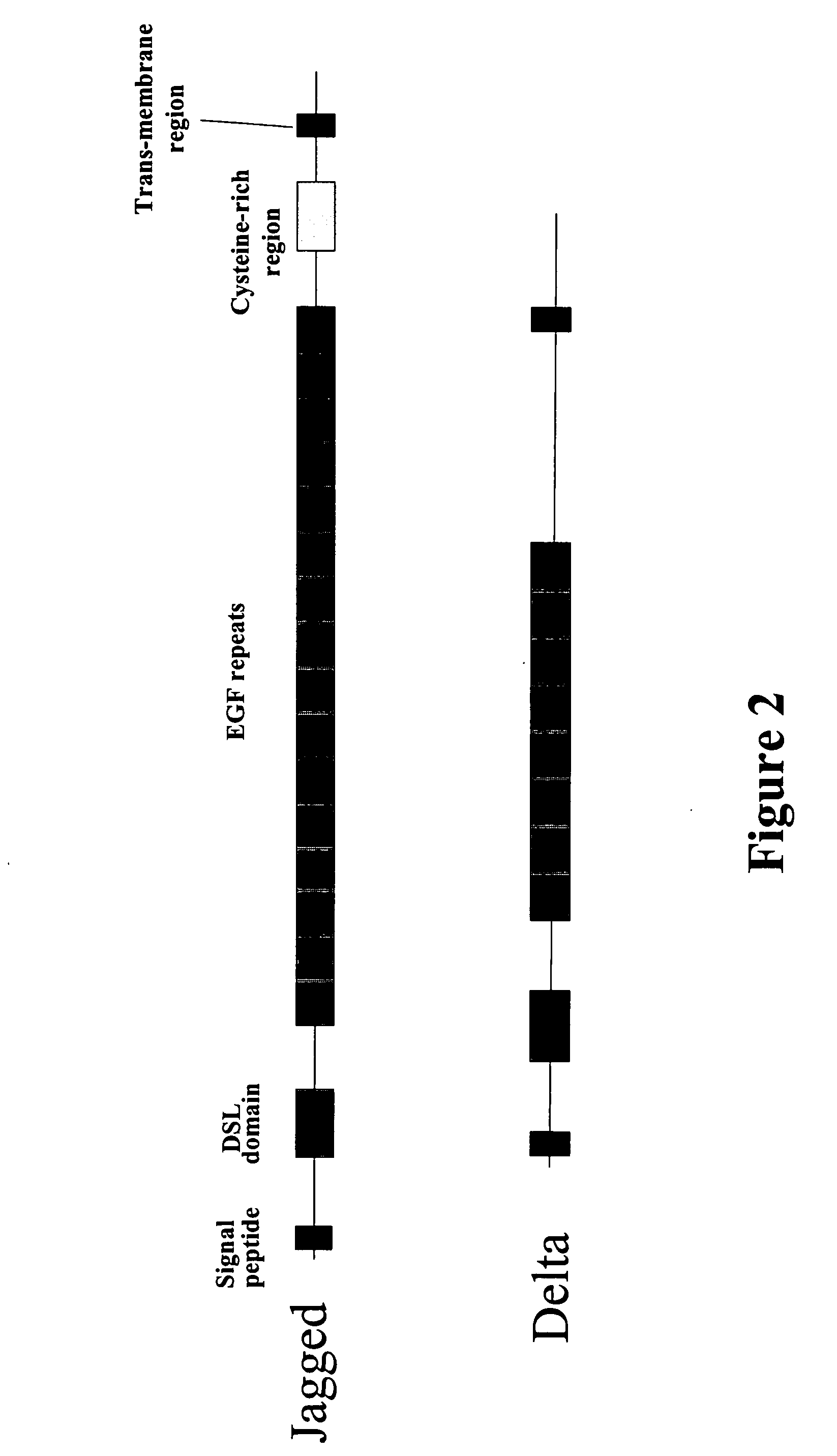
Therapeutic use of modulators of notch
InactiveUS20060128619A1Increasing IL- expressionLower immune responseSenses disorderNervous disorderImmuno modulationTreatment use
Provided is a method for modifying IL-4 expression in a cell using a modulator of Notch signalling. Also provided are methods for generating immune modulatory cytokine profiles with increased IL-4 expression and / or increased IL-10 expression and / or reduced IL-5, IL-13 and TNFα expression. In addition, a method for increasing a TH2 immune response and / or decreasing a TH1 immune response in a cell, using a modulator of Notch signalling, is provided. Methods of treatment are also disclosed.
Owner:LORANTIS
Mucosal immunoadjuvant inducing Th1 immune response, and application thereof
InactiveCN103214554AStimulate Th1 type immune responseImprove immunityAntibody medical ingredientsHybrid peptidesBALB/cMucosal Immune Responses
The invention relates to a mucosal immunoadjuvant inducing Th1 immune response. A bioinformatics technology is applied in the invention, the spatial conformation of a ricin B-chain monoclonal anti-3E1 and Ricin-B compound which has obtained a Chinese invention patent is determined through the action of an antibody-antigen compound, and a molecular stimulation technology is utilized to reasonably determine four Ricin B identifying active regions of 3E1 and derive four nucleotide sequences. Four GFP-nRTB fusion proteins are obtained through a molecular cloning technology. Results of experiments that the four proteins are used to immunize BALB / C mice through a sublingual way three times show that a P1-GFP protein in the four proteins excites Th1 immune response, and a high-level IgG2a antibody is generated. The capability and the possible molecular mechanism of the targeting GFP mucosal immune response of the P1-GFP protein are discussed. The mucosal immunoadjuvant inducing Th1 immune response, which is screened through the above scheme, can be used for preparing vaccines, immunomodifiers or treatment medicines.
Owner:PLA NAVY GENERAL HOSIPTAL
Modified plant viruses and methods of use thereof
InactiveUS20080124358A1Reducing TH2-TypeReduced responseAntibacterial agentsOrganic active ingredientsAntigenPlant virus
Owner:PFENEX
Vaccine carrier based on aluminum hydroxide nanoparticles
PendingCN107496915AReduce aluminum contentNo inflammationPharmaceutical non-active ingredientsImmunological disordersDendritic cellAluminium hydroxide
The invention relates to a vaccine carrier based on aluminum hydroxide nanoparticles. A PEG derivate material and an aluminum salt are compounded into nanoparticles. The vaccine carrier not only remains the property of powerful Th2 humoral immunologic adjuvant of the aluminum salt but also can be effectively in vivo transferred to drainage lymph gland and can be easily absorbed by dendritic cells (DC), can perform effective cross presentation, can induce cellular immunologic response and has strong Th1 immune response.
Owner:SICHUAN UNIV
Anti-neutrophil activity on innate immune response
ActiveUS20190038616A1Suture equipmentsOrganic active ingredientsInnate immune systemNeutrophil granulocyte
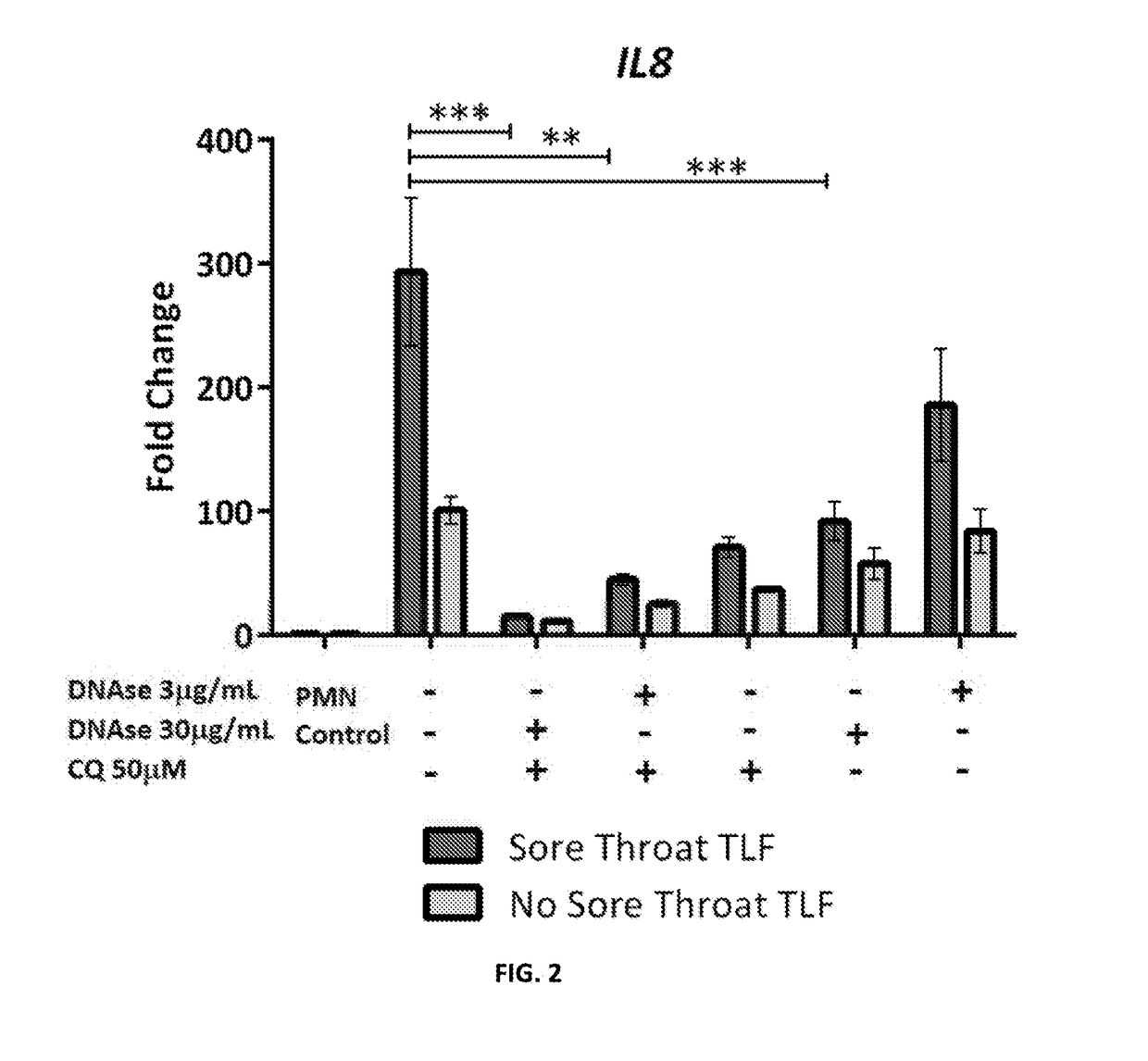
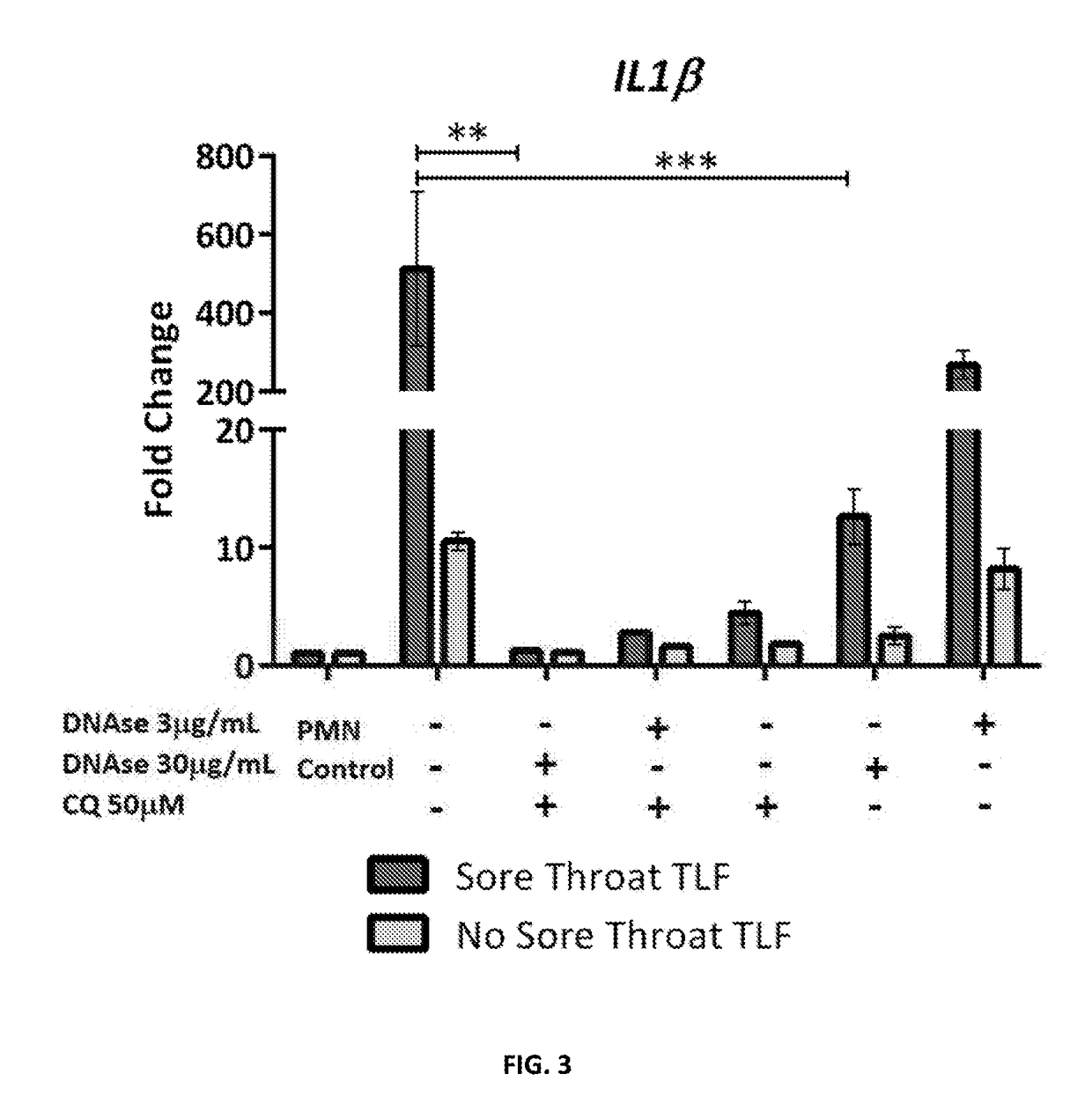
Disclosed are compositions, medical devices and methods for ameliorating sterile injury due object use in a subject in need thereof. More particularly, the present disclosure relates to compositions including N-Acetylcysteine and an aminoquinoline. The present disclosure also relates to compositions including an endonuclease and an aminoquinoline. The present disclosure also relates to medical devices including a coating comprising N-Acetyl-cysteine, an aminoquinoline, an endonuclease, and combinations thereof.
Owner:WASHINGTON UNIV IN SAINT LOUIS
Staphylococcus aureus fusion protein and protein expression vector and purification method thereof
InactiveCN109942719ABacteriaMicroorganism based processesPurification methodsColony-stimulating factor
The invention discloses fusion protein containing staphylococcus aureus SSL6 protein and a mouse granulocyte-macrophage colony stimulating factor (GM-CSF) and an expression vector and purification method thereof. The fusion protein is composed of the SSL6 (aglucon of CD47) and the GM-CSF. On one hand, it is hopeful to achieve the anti-tumor immunity regulating effect of the SSL6; on the other hand, it is hopeful to achieve the immunity optimization effect of a GM-CSF-mediated tumor microenvironment. Maturation of dentritic cells is induced, a Th1 immune response and a CD8+CTL killing effect are promoted, and thus the anti-tumor effect is synergistically achieved.
Owner:THE SECOND AFFILIATED HOSPITAL ARMY MEDICAL UNIV
Method of inducing immune tolerance
InactiveUS7744863B1Peptide/protein ingredientsImmunoglobulins against cell receptors/antigens/surface-determinantsImmune toleranceCD3 Antibody
A method of inhibiting Th1 immune response in a subject in need of such treatment which comprises administering to the subject effective amounts for inhibiting Th1 immune response of an anti-CD3 monoclonal antibody and IL-5 or an analogue or mimetic thereof.
Owner:HALL BRUCE MILNE +1
Hepatitis B vaccine agonist composition and application thereof
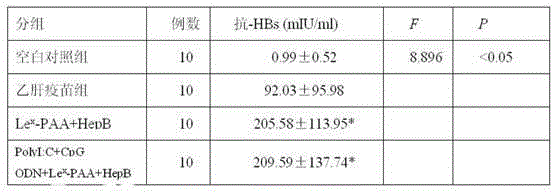
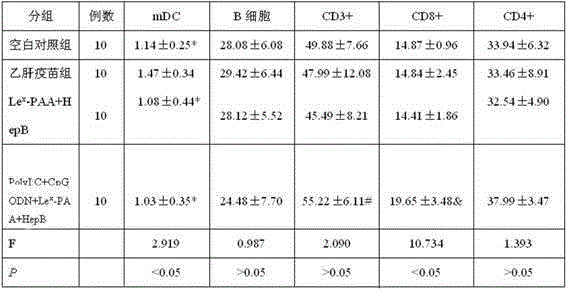
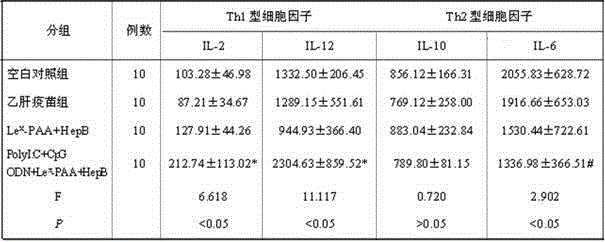
ActiveCN105194666AImprove antigen presentation functionRaise the level of immune responseAntiviralsAntibody medical ingredientsNucleotideBiotin
The invention discloses a hepatitis B vaccine agonist composition and application thereof, and relates to a hepatitis B vaccine. The hepatitis B vaccine agonist composition comprises Lewisx-Polyacrylamide-biotin, polyinosinic acid-polycytidylic acid, and oligodeoxynucleotide, wherein the component of the Lewisx-Polyacrylamide-biotin is 30 kd polyacrylamide containing 5 mol% of biotin and 20 mol% of a carbohydrate; the sequence of the oligodeoxynucleotide is 5'-tcgacgttcgtcgttcgtcgttc-3', and the whole process is subjected to phosphorothioate modification. Through agonists of three receptors DC-SIGN, TLR3 and TLR9, high expression of corresponding acceptors are stimulated, so that the antigen presenting function of DC is improved, and Th1 immune response of a body is promoted.
Owner:SHANXI MEDICAL UNIV
Pneumococcus conjugate vaccine and preparation method thereof
InactiveCN106668853AImproving immunogenicitySmall doseAntibacterial agentsBacterial antigen ingredientsConjugate vaccineMicrosphere
Owner:BRAVOVAX
A mucosal immune adjuvant for inducing th1-type immune response and its application
InactiveCN103214554BStimulate Th1 type immune responseImprove immunityAntibody medical ingredientsHybrid peptidesMucosal Immune ResponsesBALB/c
Owner:PLA NAVY GENERAL HOSIPTAL
Immune-modulating compounds
PendingUS20190389929A1Eliminate the effects ofBlock immunosuppressive functionImmunoglobulin superfamilyPeptide/protein ingredientsDiseaseTh1 immune response
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
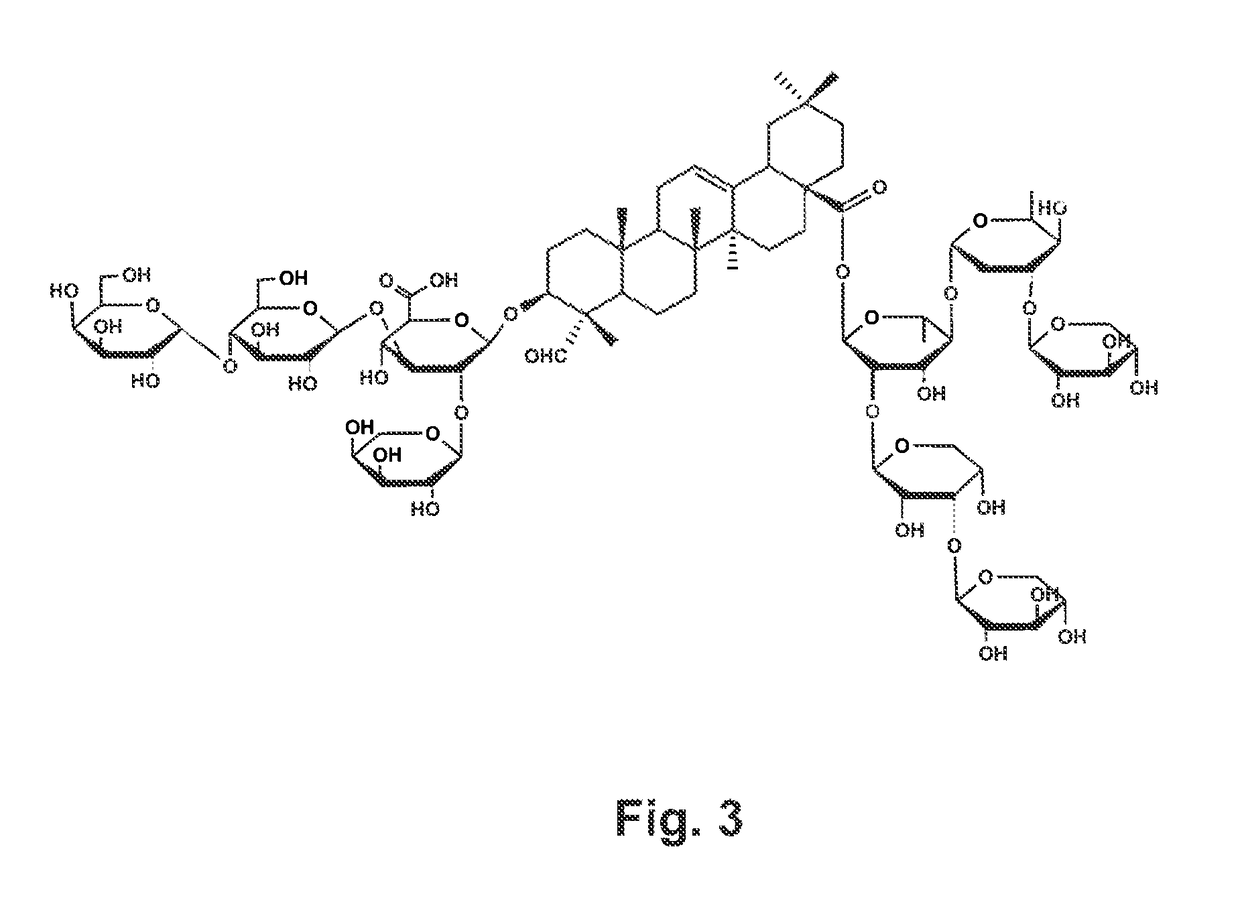
Vaccine formulations comprising quillaja desacylsaponins and beta amyloid peptides or tau protein to induce a Th2 immune response
ActiveUS10195257B2Preventing and delaying onsetImproved prognosisNervous disorderPeptide/protein ingredientsAdjuvantAmyloid beta
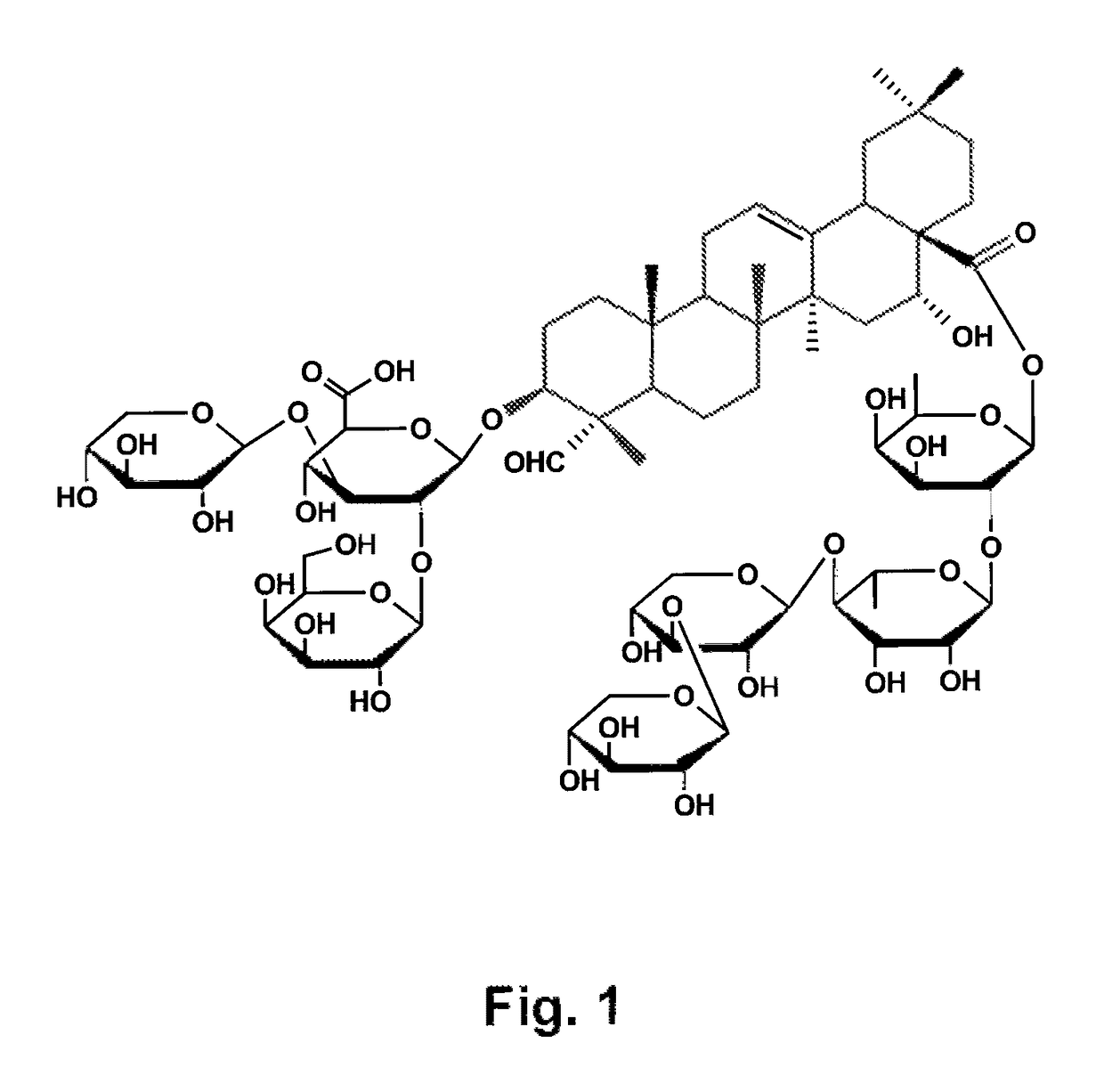
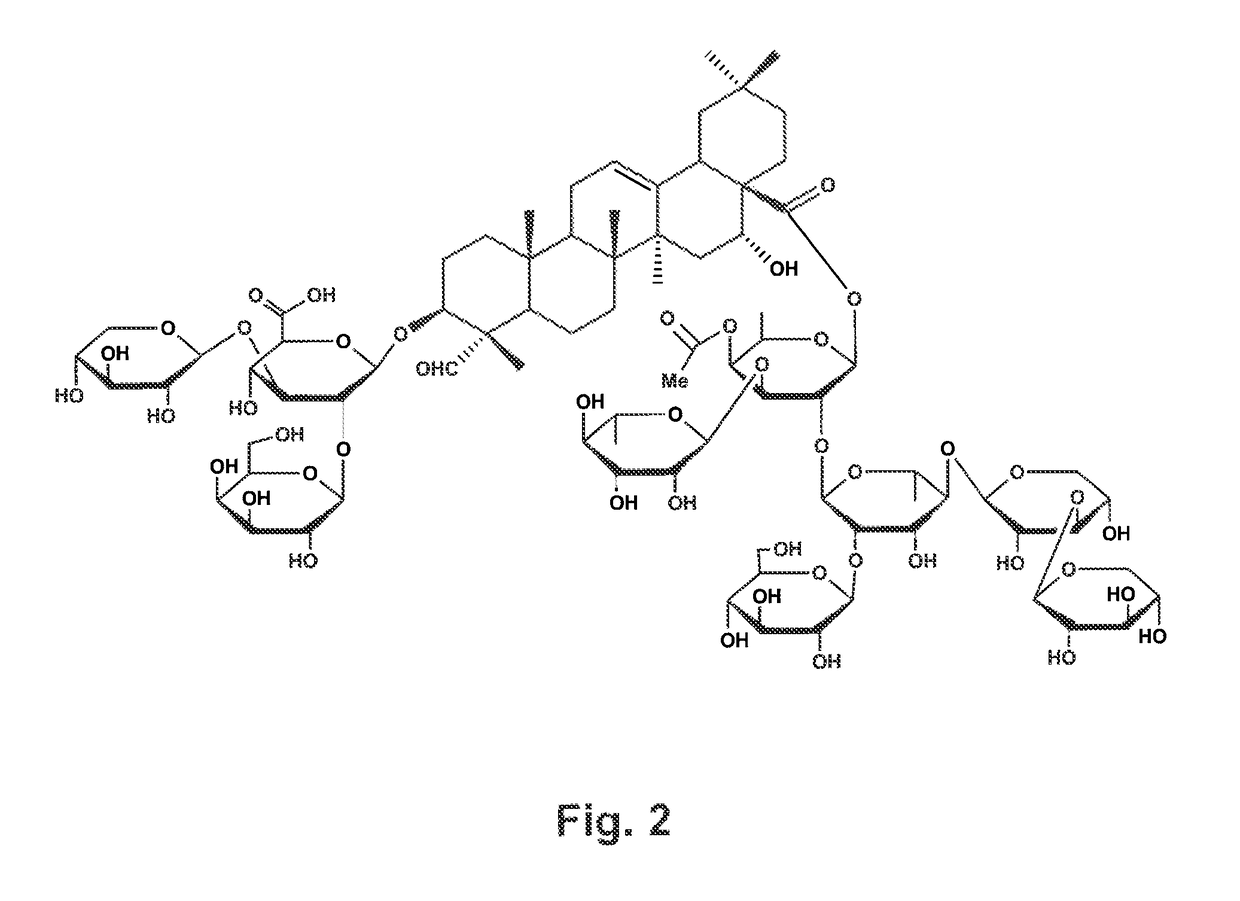
The present invention relates to compositions and methods for the prevention and treatment of neurodegenerative diseases, such Alzheimer's disease, that are caused by misfolding, aggregating proteins. The compositions and methods of the present invention comprise a vaccine formulation comprising an antigen selected from the group consisting of i) amyloid-β or a peptide that has in its amino acid sequence part of the amyloid-β amino acid sequence, ii) hyperphosphorylated tau protein or one of its hyperphoshorylated peptides, or iii) a combination of antigens derived from groups i) and ii) and that are formulated with a non-acylated or deacylated, natural or synthetic, bidesmosidic triterpene glycoside carrying an aldehyde or ketone group, which acts as an adjuvant or immune agonist. These vaccine formulations are capable of stimulating a Th2 immunity or antibody response against antigens such as amyloid-β and tau derived antigens, but not a Th1 immune response.
Owner:QANTU THERAPEUTICS
Tuberculosis gene vaccine based on T cell epitope as well as preparation method and use thereof
InactiveCN101451145BActivate immune responseDoes not affect the spatial structureAntibacterial agentsGenetic material ingredientsTreating tuberculosisSpecific antibody
Owner:FUDAN UNIV
Use as immune enhancer or pharmaceutical composition for treatment of dementia, comprising phytosphingosine-1-phosphate or derivative thereof
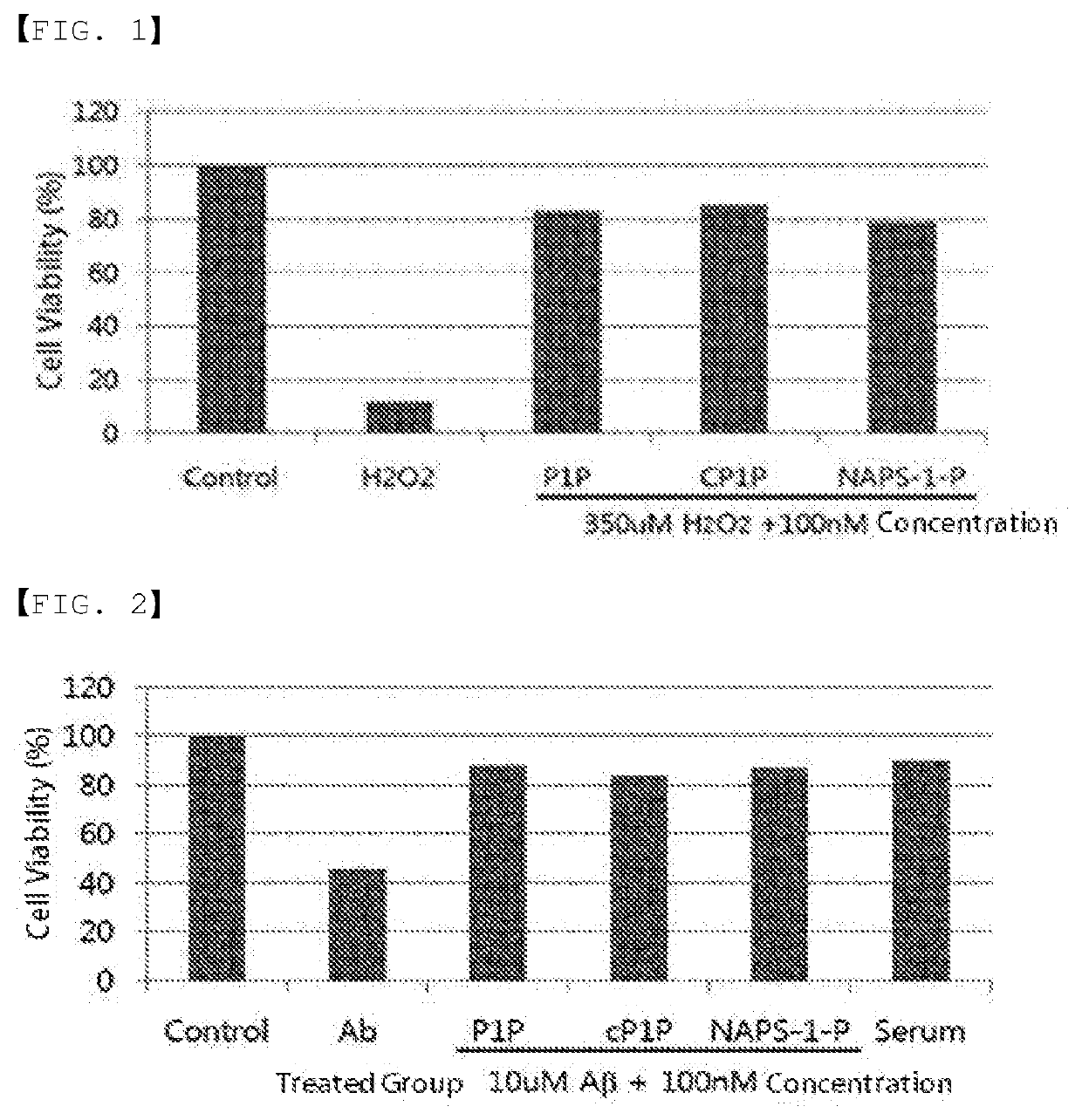
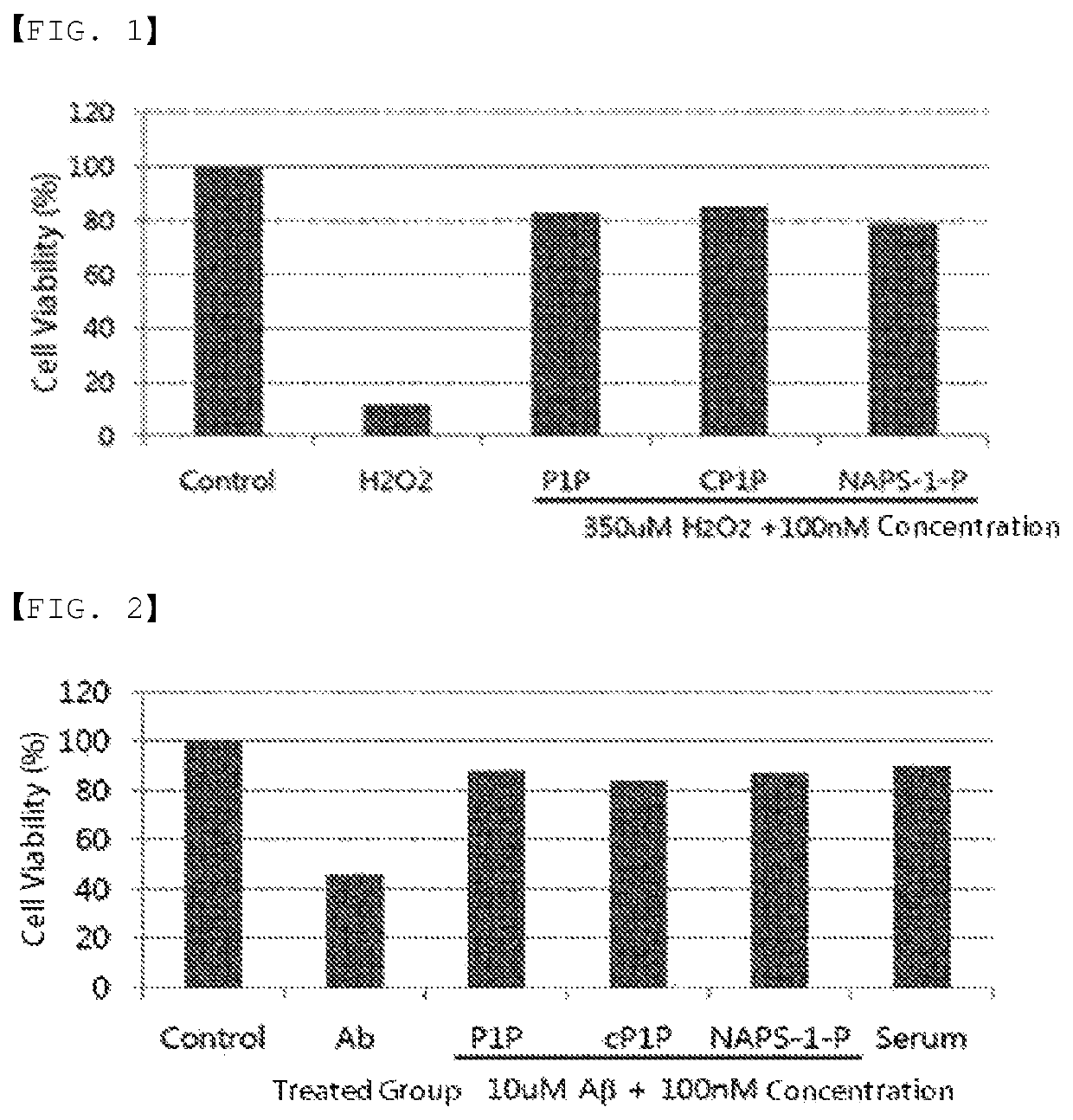
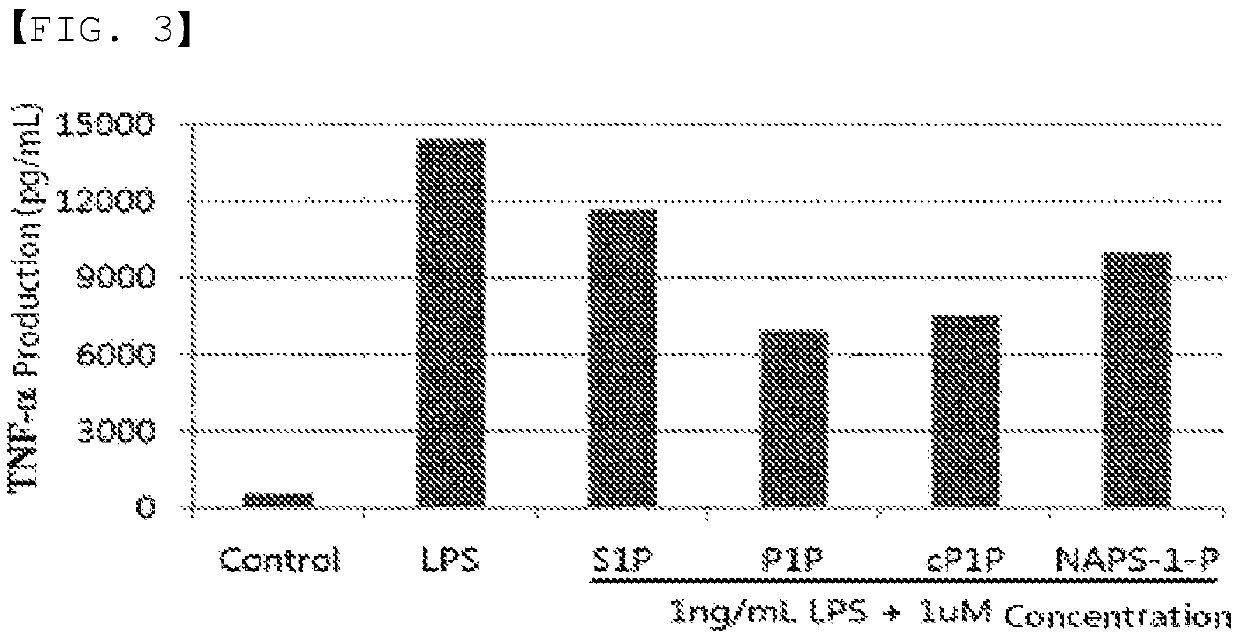
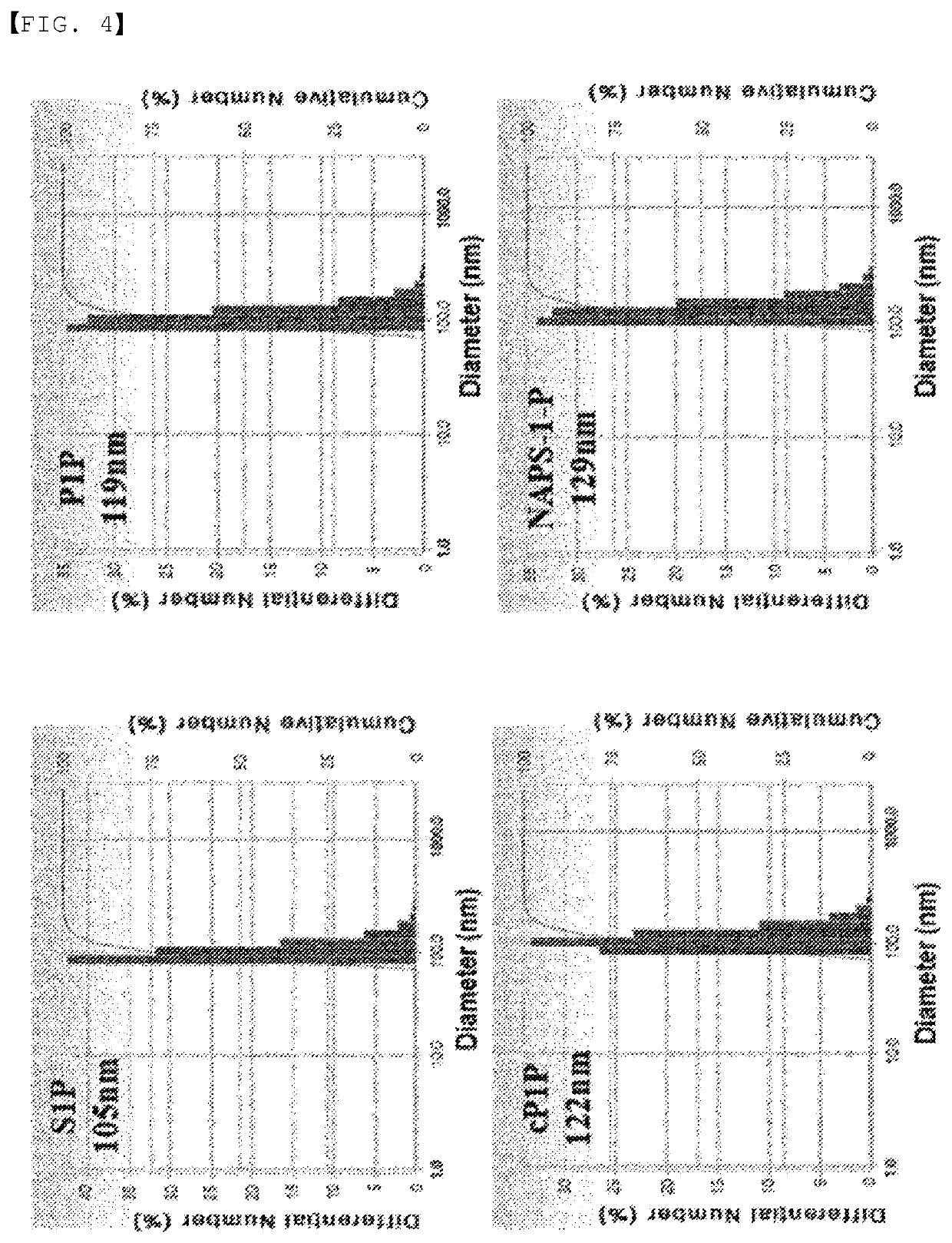
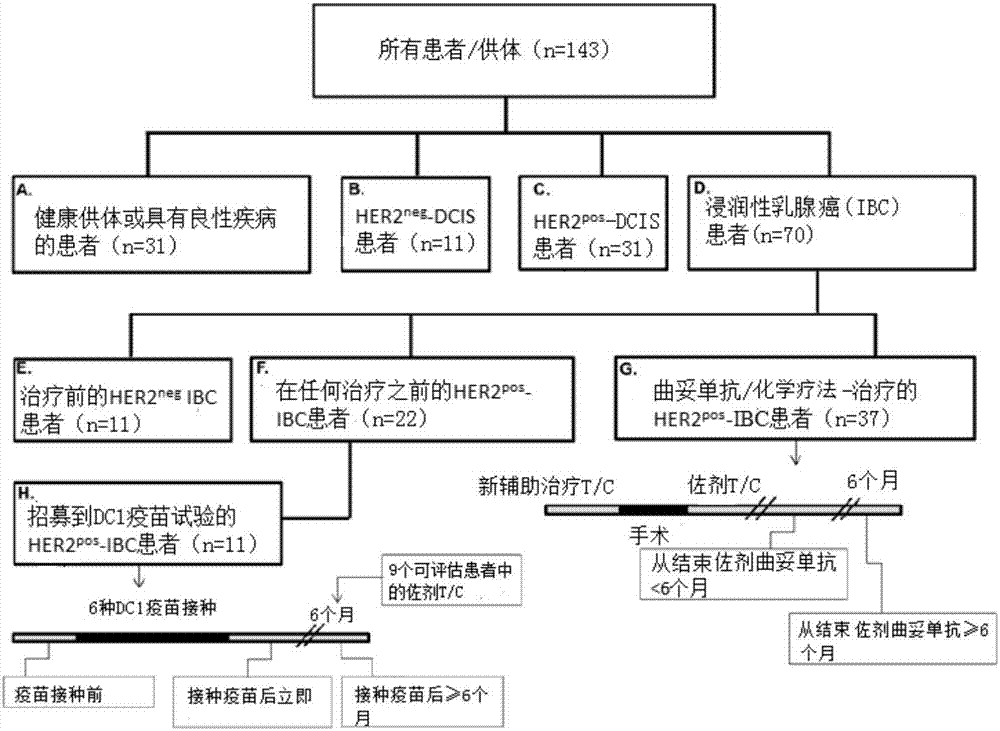
ActiveUS20200046829A1Inducing cellular immune responseEasy to useOrganic active ingredientsNervous disorderNeuron cell deathAntiendomysial antibodies
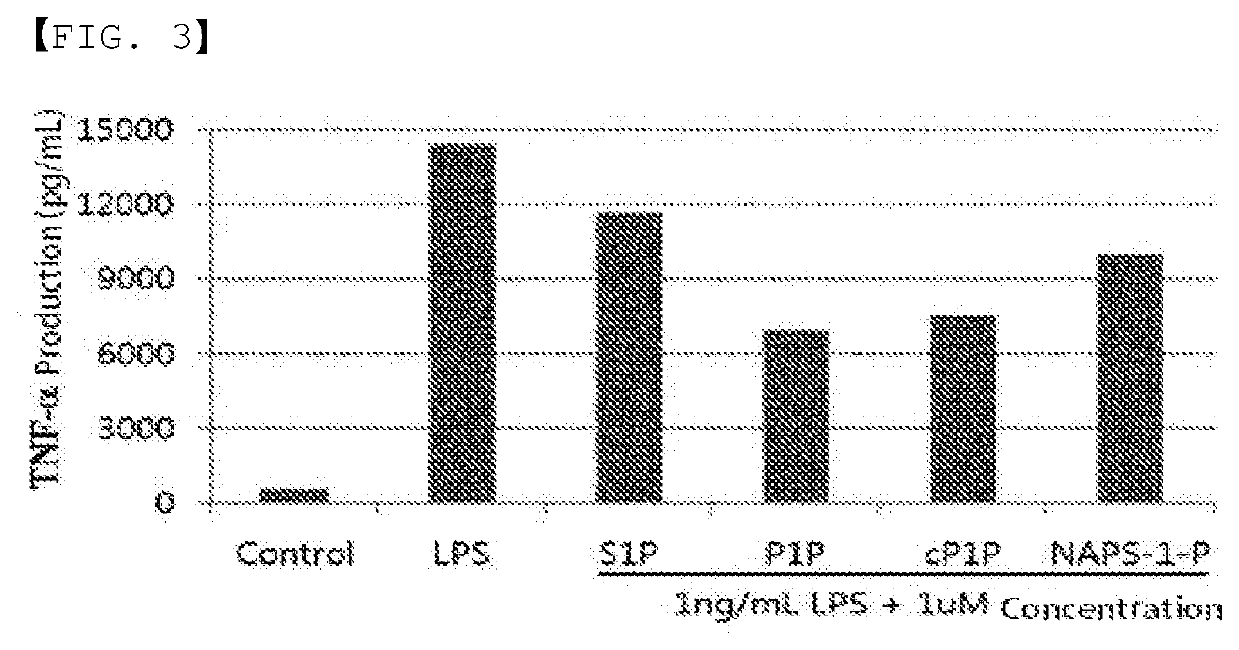
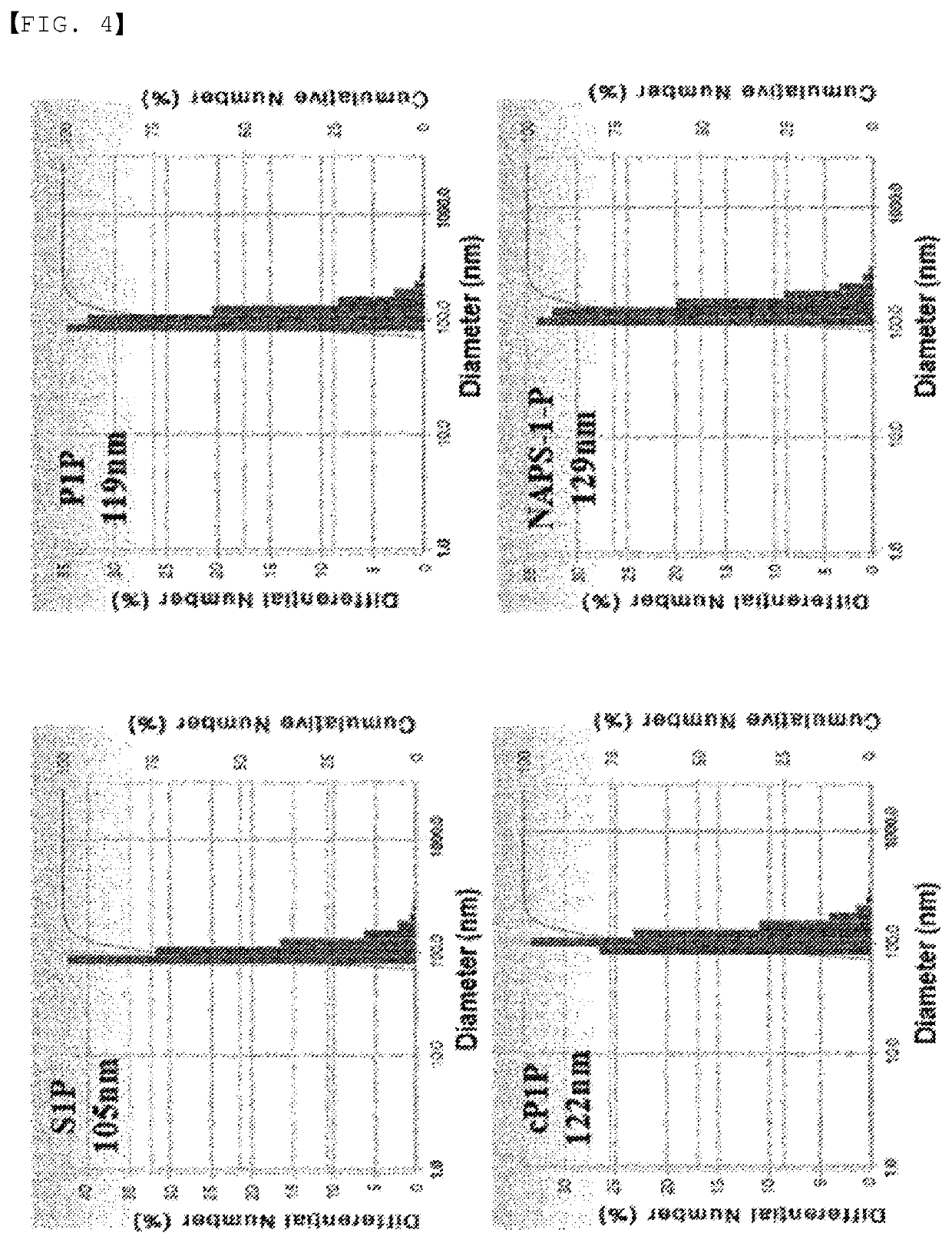
Disclosed in the present invention are a use as an immune enhancer of phytosphingosine-1-phosphate (P1P), O-cyclic P1P (cP1P), N-acetylphytosphingosine-1-phosphate (NAPS-1-P), and pharmaceutically acceptable salts thereof, and a pharmaceutical composition or vaccine for treating dementia, comprising such substance on the basis of a neuronal cell death inhibitory effect. The substance according to the present invention exhibits an effect of enhancing Th2 immune response and inhibiting Th1 immune response and has an effect of protecting neurons as well, and thus can be useful as an immune enhancer to help the production of antibodies for the development of a dementia vaccine, as well as an agent for preventing or treating dementia.
Owner:AXCESO BIOPHARMA CO LTD
Immune-modulating compounds
Owner:COUNCIL OF THE QUEENSLAND INST OF MEDICAL RES
Vaccine for controlling persistent infection of hepatitis B virus
InactiveCN102038948BExcellent adjuvant effectPromote maturityAntiviralsAntibody medical ingredientsAdjuvantHepatitis B Surface Antigens
The invention belongs to the biotechnology field, and relates to a vaccine for controlling the persistent infection of hepatitis B virus. According to the invention, a Hansenula polymorpha cell that is heat inactivated and expresses hepatitis B surface antigen is adopted as the hepatitis B vaccine, wherein the HBsAg is an antigen part of the vaccine and the Hansenula polymorpha cell is an adjuvant part of the vaccine. Animal immunity experiment shows that: the vaccine can induce the aggregation of immune cells to the spleen, promote the maturation of DCs, and both induce Th1 immune response and enhance Th2 immune response in mice, including total IgG, IgG1, IgG2a, specific CTL activity, and the production ability of antigen-specific IFN-gama. The invention can make up for the deficiency of the impossibility of inducing Th1 immune response for traditional vaccines that take aluminium hydroxide as an adjuvant, and is helpful to the control of the persistent infection of hepatitis B virus.
Owner:FUDAN UNIV
Use as immune enhancer or pharmaceutical composition for treatment of dementia, comprising phytosphingosine-1-phosphate or derivative thereof
ActiveUS11000587B2Inducing cellular immune responseEasy to useOrganic active ingredientsNervous disorderNeuron cell deathAntiendomysial antibodies
Disclosed in the present invention are a use as an immune enhancer of phytosphingosine-1-phosphate (P1P), O-cyclic P1P (cP1P), N-acetylphytosphingosine-1-phosphate (NAPS-1-P), and pharmaceutically acceptable salts thereof, and a pharmaceutical composition or vaccine for treating dementia, comprising such substance on the basis of a neuronal cell death inhibitory effect. The substance according to the present invention exhibits an effect of enhancing Th2 immune response and inhibiting Th1 immune response and has an effect of protecting neurons as well, and thus can be useful as an immune enhancer to help the production of antibodies for the development of a dementia vaccine, as well as an agent for preventing or treating dementia.
Owner:AXCESO BIOPHARMA CO LTD
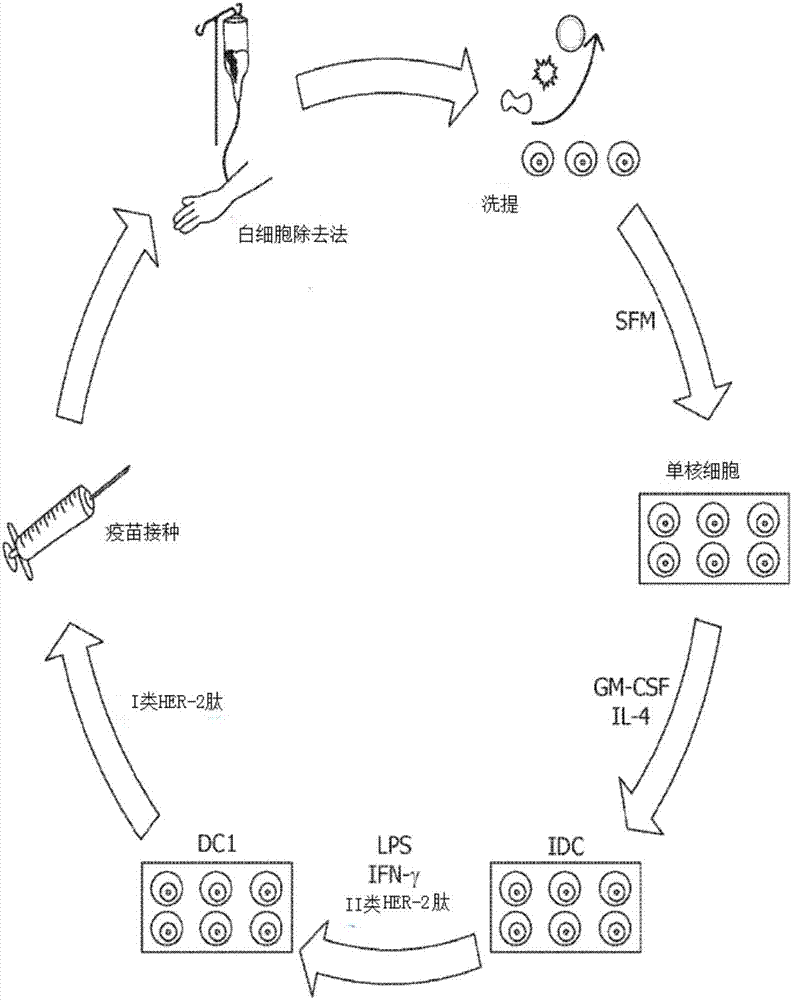
Methods for monitoring CD4+ T-helper type 1 response in cancer and immune restoration
InactiveCN107533065APeptide/protein ingredientsMammal material medical ingredientsHER2 Positive Breast CancerDc vaccination
A method for diagnosing or treating a mammalian subject having, or at risk of developing cancer, comprises: generating a circulating anti-cancer CD4+ Th1 response from antigen presenting cells or their precursors and CD4+ T-cells from a sample of the subject's blood which causes secretion of interferon-gamma ('IFN-[gamma]'); and detecting the anti-cancer CD4+ Th1 response to determine if the response is depressed. A method for restoring HER2-specific CD4+ Th1 immune response in a HER2-positive breast cancer patient in need thereof, comprises: administering to the patient a therapeutically effective amount of a dendritic cell ('DC') vaccine comprising autologous DCs pulsed with HER2-derived MHC class II binding peptides ('DC vaccination') to elevate the patient's anti-HER2 CD4+ Th1 response; and measuring said anti-HER2 Th1 response of the patient pre- and post-DC vaccination to determine the amount of increase in the response.
Owner:布莱恩 J 赫尔尼奇
Tuberculosis mucosa gene vaccine assembled by using chitosan oligosaccharide delivery system and preparation and application of tuberculosis mucosa gene vaccine
InactiveCN102580115AEffective immune protection against tuberculosisSafe and non-toxicAntibacterial agentsBacterial antigen ingredientsAntigenOligosaccharide
The invention discloses a tuberculosis mucosa gene vaccine assembled by using a chitosan oligosaccharide delivery system. The vaccine is formed by copolymerizing, crosslinking and compounding a chitosan oligosaccharide and a tuberculosis antigen encoding plasmid; a full-length gene of a heat shock protein HSP65 derived from a mycobacterium tuberculosis H37Rv strain is inserted into the tuberculosis antigen encoding plasmid; and four T cell epitope genes, namely, ESAT-6189-228, Ag85A369-405, CFP10162-207 and Ag85B420-459 derived from the mycobacterium tuberculosis H37Rv strain are inserted into the HSP65 full-length gene. The invention further discloses a preparation method and an application of the tuberculosis mucosa gene vaccine. The tuberculosis mucosa gene vaccine disclosed by the invention can be used for inducing tuberculosis specific serum antibodies and systemic Th1 immune response, can be used for inducing lung mucosa locally-enhanced tuberculosis specific secretion type SIgA and IFNgama+Th1 response, and has a remarkably superior effect to that of a naked pECANS gene vaccine.
Owner:FUDAN UNIV
Tuberculosis mucosa gene vaccine assembled by using chitosan oligosaccharide delivery system and preparation and application of tuberculosis mucosa gene vaccine
InactiveCN102580115BEffective immune protection against tuberculosisSafe and non-toxicAntibacterial agentsBacterial antigen ingredientsAntigenWhole body
Owner:FUDAN UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com