Mucosal immunoadjuvant inducing Th1 immune response, and application thereof
A mucosal immune adjuvant and immune response technology, applied in the direction of medical preparations containing active ingredients, the use of carriers to introduce foreign genetic material, hybrid peptides, etc., can solve the problem of poor immune protection, short duration of immune response, antigenic Weak immunogenicity and other issues, to achieve good social and economic benefits, good immune effect, and broad market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0072] Example 1 Determination of RTB and cell specific binding active site
[0073] 1. Use molecular simulation to build 3E1 light and heavy chain antibody spatial conformation
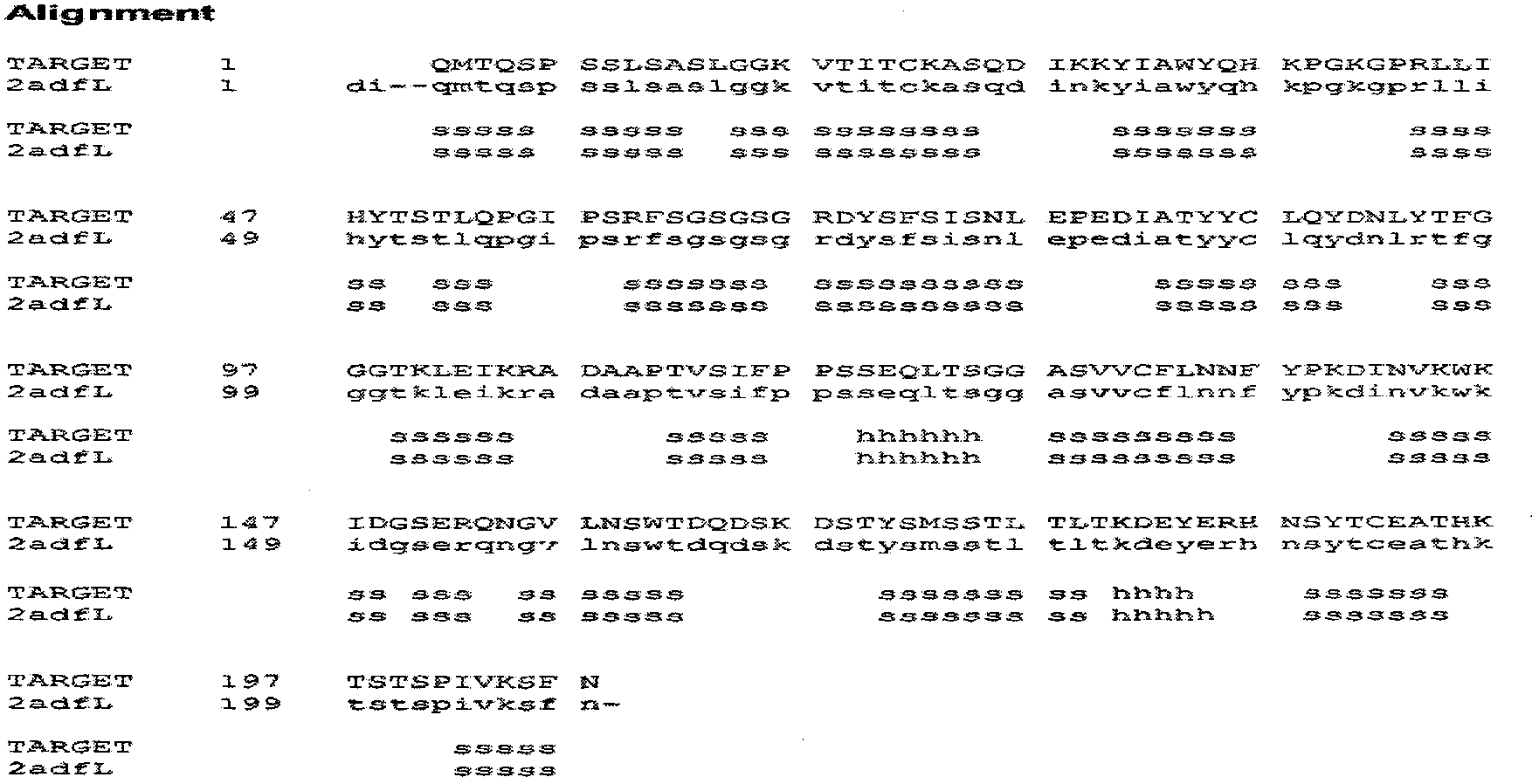
[0074] The amino acid sequence of the heavy chain of the 3E1 antibody with independent intellectual property rights is as follows: (see sequence 1 in the sequence table)
[0075] S G A E L M K P G A S V K I S C K A T G Y T F S S Y W I E W I K Q R P G H G L E W I G D I L P G SG S T N Y N E K F K G K A T F T A D T S S N T A Y M Q L S S L T S E D S A V Y Y C S R S F Y Y N Y D GA Y F A Y W G Q G T L V T V S A A K T T P P S V Y P L A P G S A A Q T N S M V T L G C L V K G Y F P EP V T V T W N S G S L S S G V H T F P A V L Q S D L Y T L S S S V T V P S S T W P S E T V T C N V A H PA S S T K V D K K I V P R D C
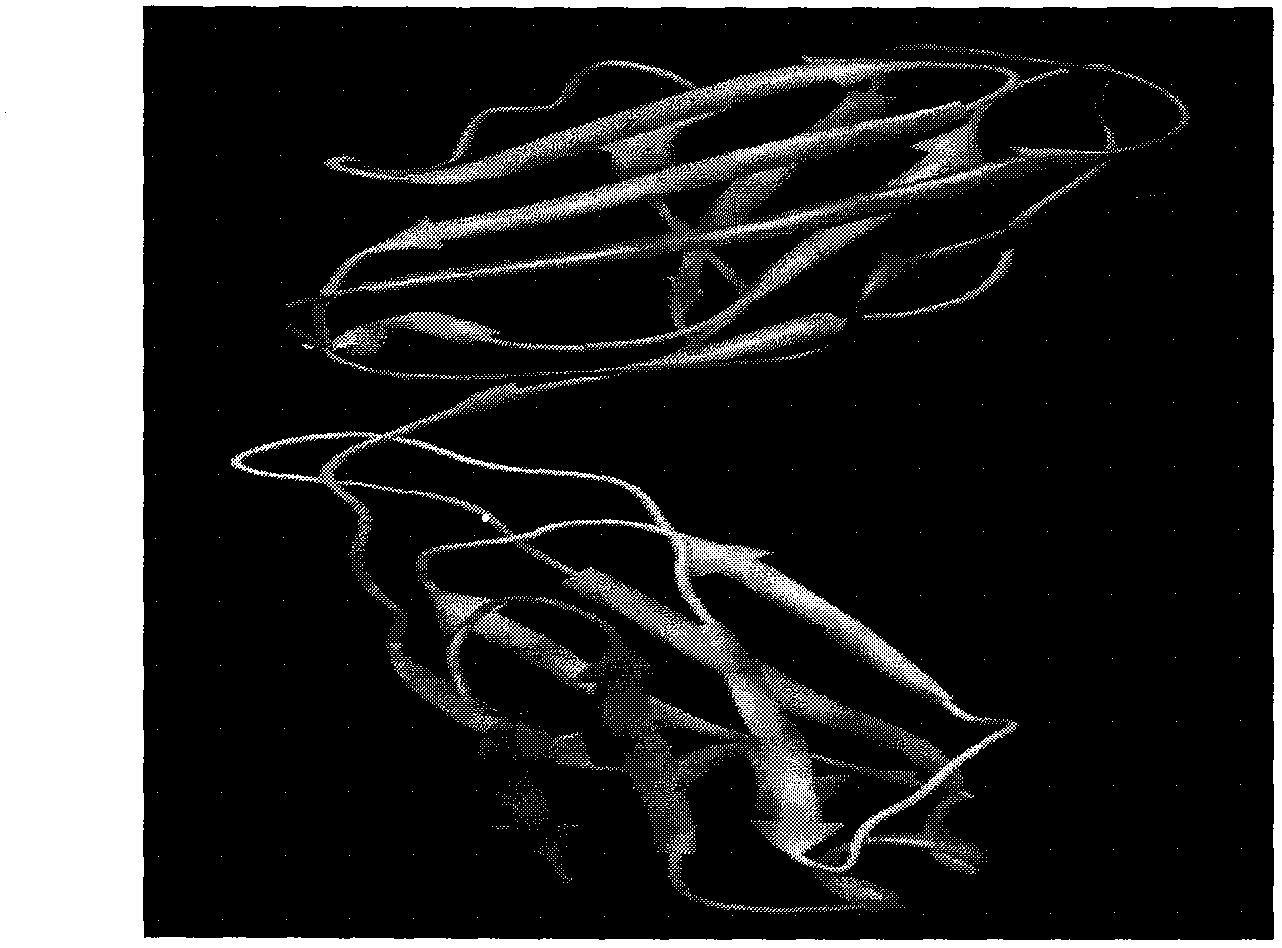
[0076] Select a template protein with high homology (PDB library number: 2V7H), perform sequence alignment, and the result ( figure 1 .). The conformation of antibody heavy chain (VH+CH1) obtained by mole...
Embodiment 2
[0090] Example 2. Construction, expression, purification and identification of GFP-nRTB fusion protein prokaryotic expression vector
[0091] 1. Material
[0092] PCR kit, plasmid extraction kit, gel recovery kit, lysozyme Lysozyme, DNA and protein Marker are all products of Shanghai Shenggong; PET-30a(+) prokaryotic expression vector, restriction enzyme Nco I, EcoRV, T4 DNA ligase and pGEM-T Easy were purchased from Promega. The PCR primer synthesis and DNA sequencing were completed by Shanghai Boya Biotechnology Co., Ltd.
[0093] Two, method results
[0094] 1. Construction and design of prokaryotic expression vector ( Figure 7. Figure 8. )
[0095] Use PET-30a(+) prokaryotic expression vector. After analysis with BIOSUN software, the restriction sites Nco I and EcoRV were selected. The protein is expressed from ATG, and the C-terminal of the recombinant protein has a stop codon.
[0096] 2. Design PCR primers according to the target gene sequence.
[0097] The small peptide and ...
Embodiment 3
[0117] Example 3. Preliminary evaluation of mucosal immune effect of GFP-nRTB fusion protein
[0118] 1. Material
[0119] IgG, IgG1, IgG2a, IgG2b, and IgG3 monoclonal antibodies are all products of Sigma. HRP-labeled goat anti-mouse antibody (GAM-HRP) is produced by Boaosen Biotechnology Co., Ltd.
[0120] Two, method results
[0121] 1. Preliminary evaluation of mucosal immune effect of GFP-nRTB fusion protein
[0122] The four fusion proteins and 50μg of GFP purified under the tongue were administered sublingually under the 0, 14, and 28 immunization procedures, and blood was taken from the tail on the 7th day after the third immunization. The serum levels of GFP IgG and immunoglobulin were detected. The levels of IgG1, IgG2a, IgG2b, and IgG3 were tested by indirect ELISA after the serum was diluted 1:100 with PH7.4 phosphate buffer. The results showed that the levels of GFP IgG, IgG2b, and IgG3 in the serum after P1-GFP immunization were not significantly different from those of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com