Hepatitis B vaccine agonist composition and application thereof
A technology of hepatitis B vaccine and agonist, which is applied in antiviral agents, medical preparations containing active ingredients, antibody medical ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 1. The ingredients used:
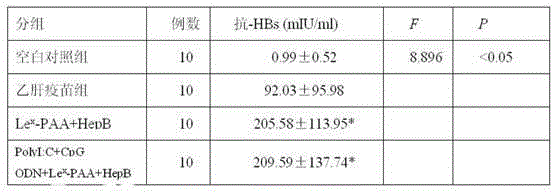
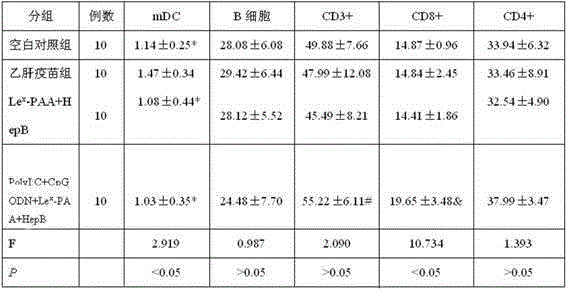
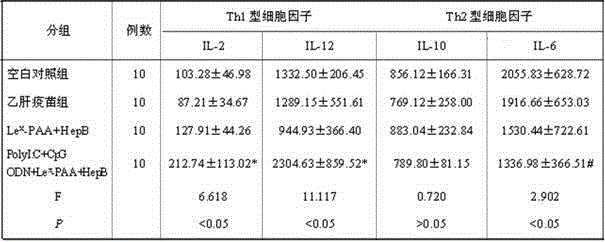
[0021] (1)Le x -PAA-biotin (Lewisx-Polyacrylamide-biotin): Lewis-polyacrylamide-biotin, produced by GlycoTech, product number 01-036, polyacrylamide with a composition of 30kd contains 5% mole of biotin and 20% Moles of carbohydrates, which do not contain stabilizers and preservatives.
[0022] (2) Recombinant Saccharomyces cerevisiae Hepatitis B Vaccine (B201109009): Produced by Shenzhen Kangtai Biological Products Co., Ltd., it is made by purifying the hepatitis B virus surface antigen (HBsAg) expressed by recombinant Saccharomyces cerevisiae and adding aluminum adjuvant. It is a milky white suspension liquid, the active ingredient is HBsAg, and the excipients are aluminum hydroxide and sodium chloride. The specification is 20μgHBsAg / ml.
[0023] (3) PolyI:C (Polyinosinicacid-polycytidylicacid): namely, polyinosinic acid-polycytidylic acid: produced by Sigma Company in the United States, white powder, the specification is 1 mg, and PolyI:...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com