Vaccine compositions and methods of use
a technology of compositions and vaccines, applied in the field of immunology, can solve the problems of difficult induction of immunodeficiency virus against proteins, difficulty in identifying distinct subtypes of viruses, and approaches that do not provide cross-reactivity to distinct subtypes of viruses, and achieve novel and cost-effective approaches, improved protection and cure, and strong increases in humoral and cell-mediated responses.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 2
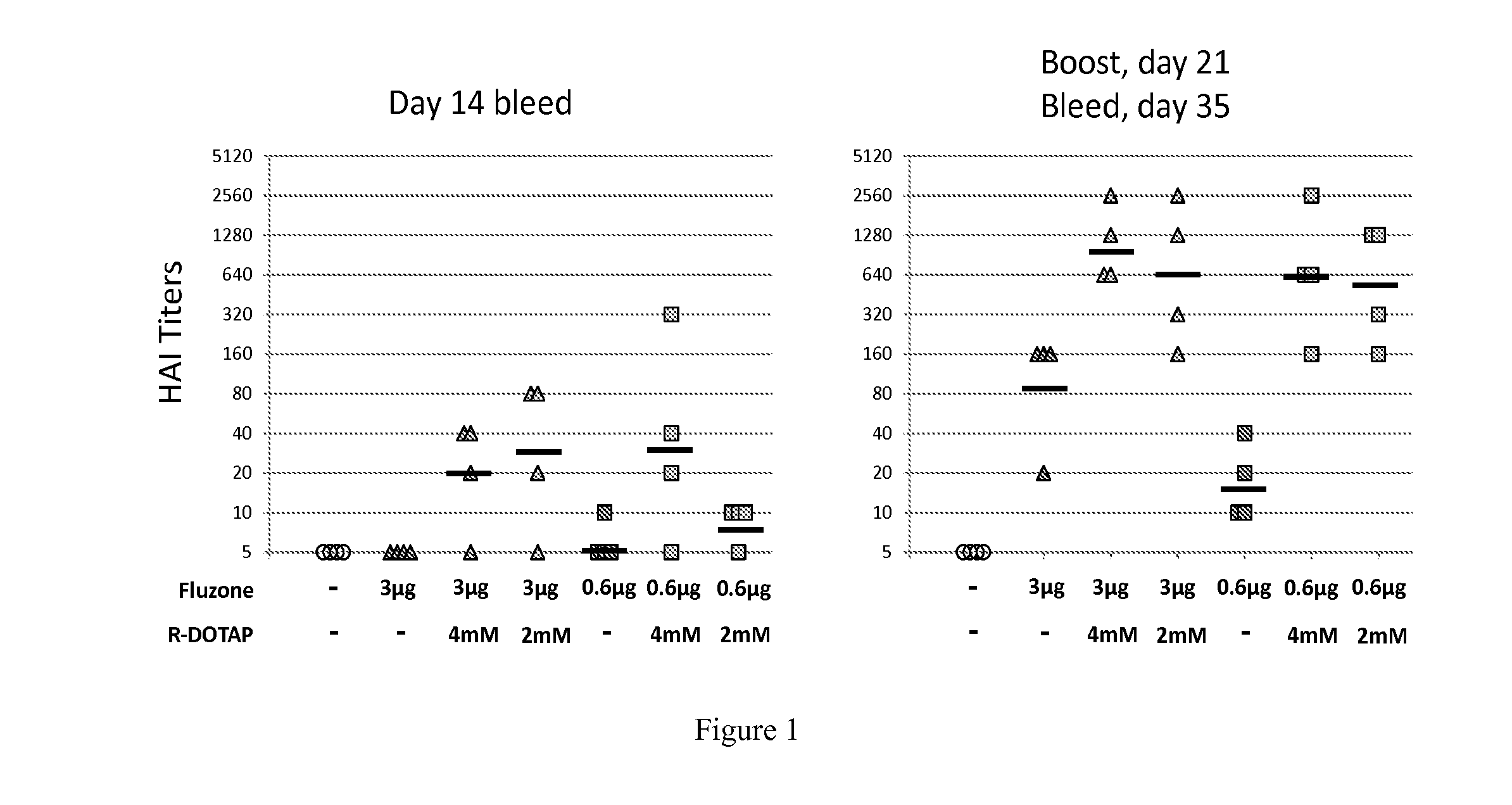
Evaluation of the Protective Potency of a Cationic Lipid-Based Influenza Vaccine: Protective Hemagglutination Inhibition Assay Against a / Perth / 16 / 2009 (H3N2)
[0389]C57BL / 6J mice were injected subcutaneously in the shaved flank with 100 μl to deliver a final dose of 3 μg or 0.6 μg of the antigen in either PBS, 4 mM R-DOTAP or 2 mM R-DOTAP. The mice were injected on day 0, then again with the identical formulation on day 21. Tail vein bleeds were performed on days 14 and 35.
[0390]Serum was stored frozen at −80° C. prior to testing. Samples were coded with respect to the treatment groups. A Hemagglutination inhibition assay was performed against the viruses A / Perth / 16 / 2009 (H3N2) to quantify the anti-influenza antibody induction and resulting protective efficacy of the vaccines.
[0391]Four mice were tested per group:
1. Naïve
2. 3 ug+PBS
3. 3 ug+4 mM R-DOTAP
4. 3 ug+2 mM R-DOTAP
5. 0.6 ug+PBS
6. 0.6 ug+4 mM R-DOTAP
7. 0.6 ug+2 mM R-DOTAP
[0392]The results are shown in FIG. 1. After the first inj...
example 3
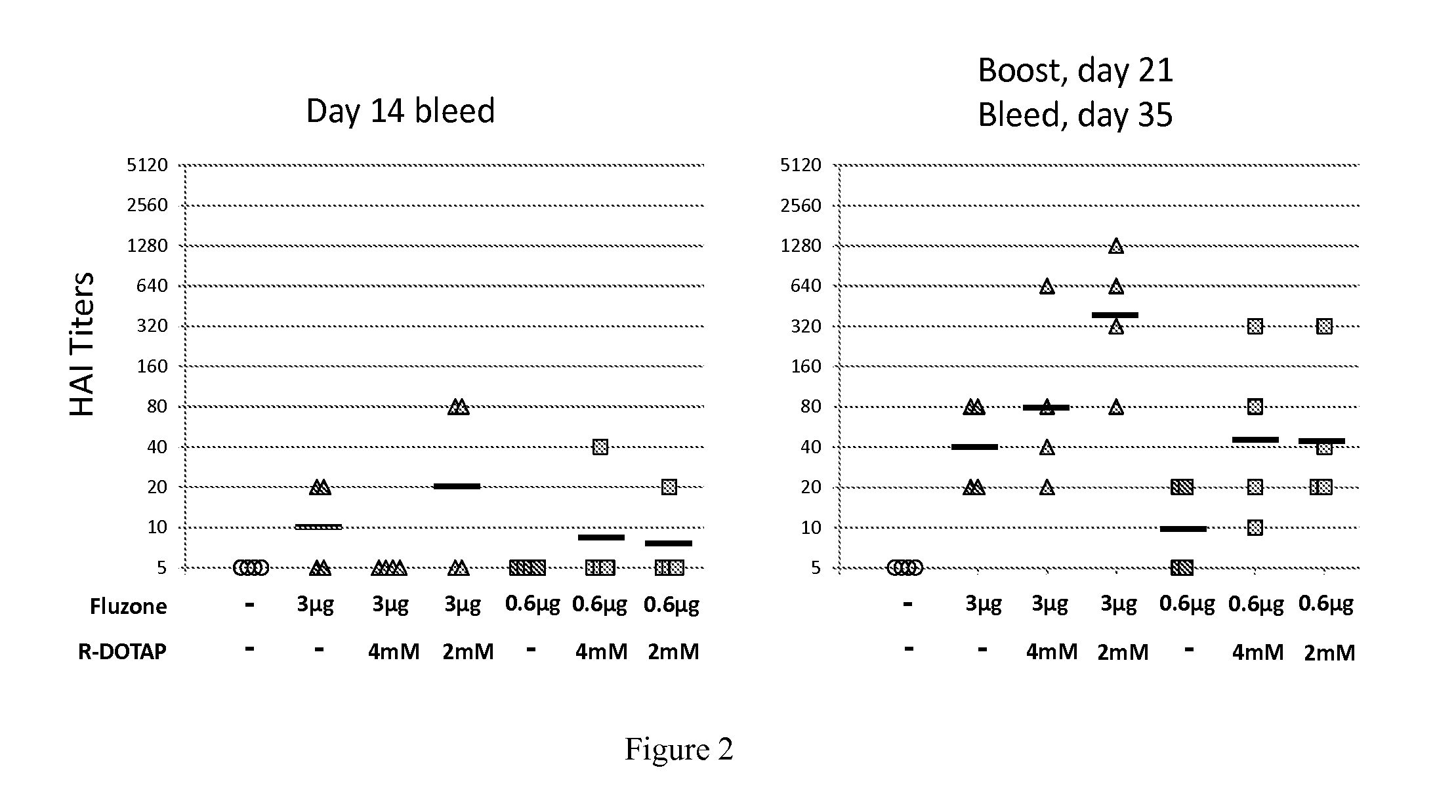
Evaluation of the Protective Potency of a Cationic Lipid-Based Influenza Vaccine: Protective Hemagglutination Inhibition Assay Against Pandemic Influenza Strain A / California / 07 / 2009 (H1N1)
[0393]C57BL / 6J mice were injected subcutaneously in the shaved flank with 100 μl to deliver a final dose of 3 μg or 0.6 μg of the antigen in either PBS, 4 mM R-DOTAP or 2 mM R-DOTAP. The mice were injected on day 0, then again with the identical formulation on day 21. Tail vein bleeds were performed on days 14 and 35.
[0394]Serum was stored frozen at −80° C. prior to testing. Samples were coded with respect to the treatment groups. A Hemagglutination inhibition assay was performed against the virus A / California / 07 / 2009 (H1N1) to quantify the antibody induction and protective efficacy of the vaccines.
[0395]Four mice were tested per group:
1. Naïve
2. 3 ug+PBS
3. 3 ug+4 mM R-DOTAP
4. 3 ug+2 mM R-DOTAP
5. 0.6 ug+PBS
6. 0.6 ug+4 mM R-DOTAP
7. 0.6 ug+2 mM R-DOTAP
[0396]The results are shown in FIG. 2. After the ...
example 4
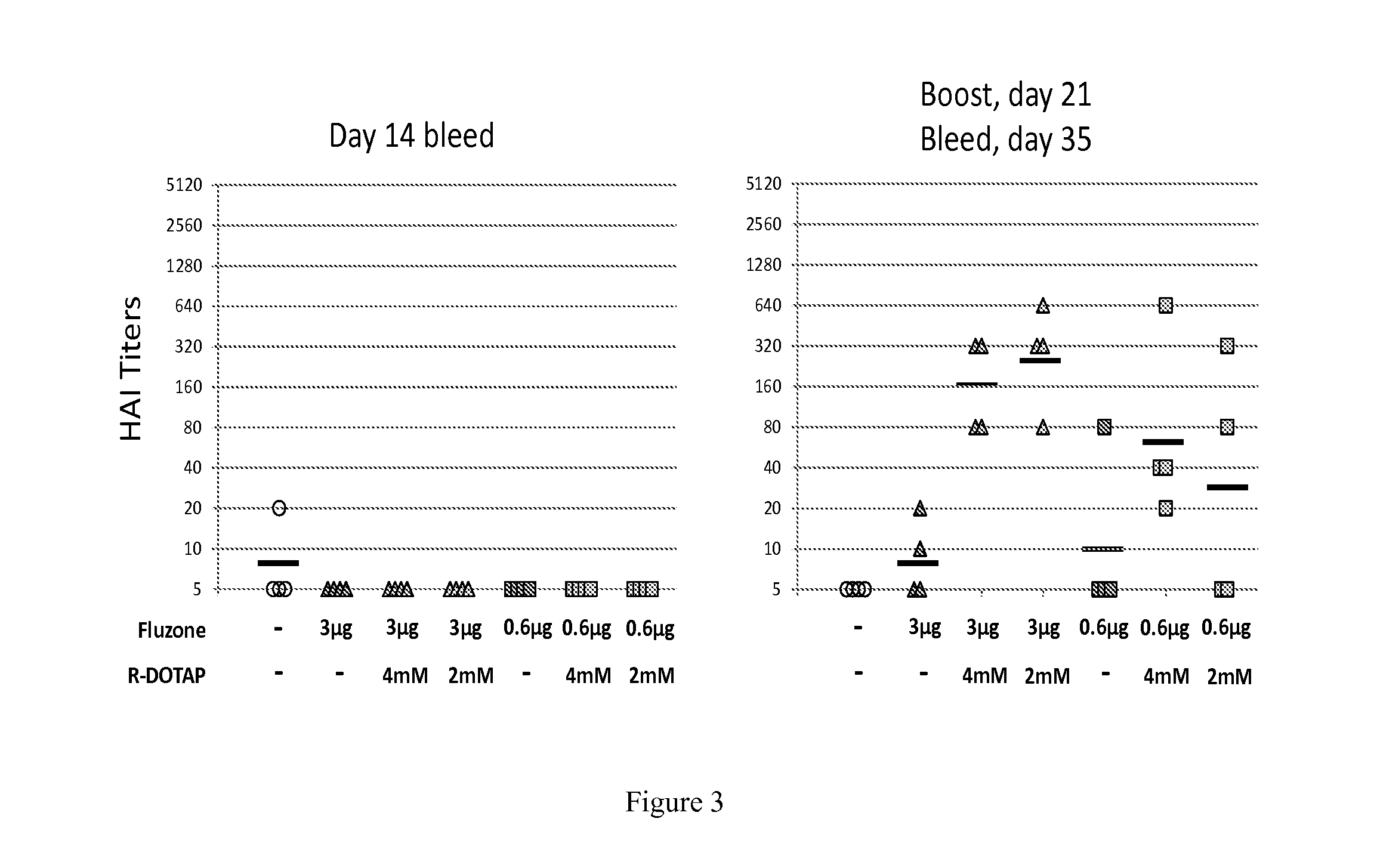
Evaluation of the Protective Potency of a Cationic Lipid-Based Influenza Vaccine: Protective Hemagglutination Inhibition Assay Against Influenza Strain B Brisbane
[0397]C57BL / 6J mice were injected subcutaneously in the shaved flank with 100 μl to deliver a final dose of 3 μg or 0.6 μg of the antigen in either PBS, 4 mM R-DOTAP or 2 mM R-DOTAP. The mice were injected on day 0, then again with the identical formulation on day 21. Tail vein bleeds were performed on days 14 and 35.
[0398]Serum was stored frozen at −80° C. prior to testing. Samples were coded with respect to the treatment groups. A Hemagglutination inhibition assay was performed against the virus B Brisbane to quantify the antibody induction and protective efficacy of the vaccines.
[0399]Four mice were tested per group:
1. Naïve
2. 3 ug+PBS
3. 3 ug+4 mM R-DOTAP
4. 3 ug+2 mM R-DOTAP
5. 0.6 ug+PBS
6. 0.6 ug+4 mM R-DOTAP
7. 0.6 ug+2 mM R-DOTAP
[0400]The results are shown in FIG. 3. After the first injection (day 14 bleed), little diff...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Immunogenicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com