Use of Anti-human sirpa v1 antibodies and method for producing Anti-sirpa v1 antibodies
A technology of antibodies and antigens, applied in chemical instruments and methods, anti-animal/human immunoglobulins, antibodies, etc., can solve problems such as lack of distinction between SIRP families
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
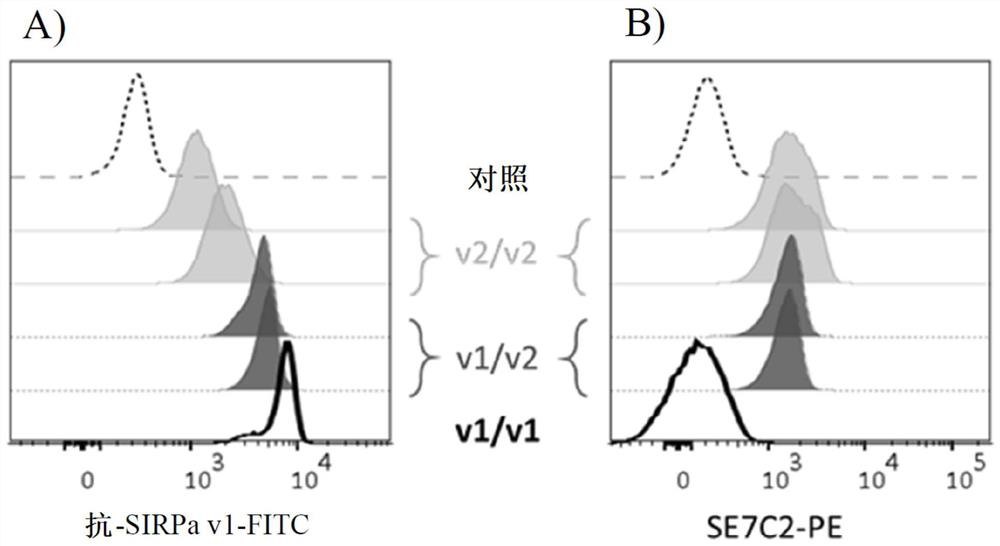
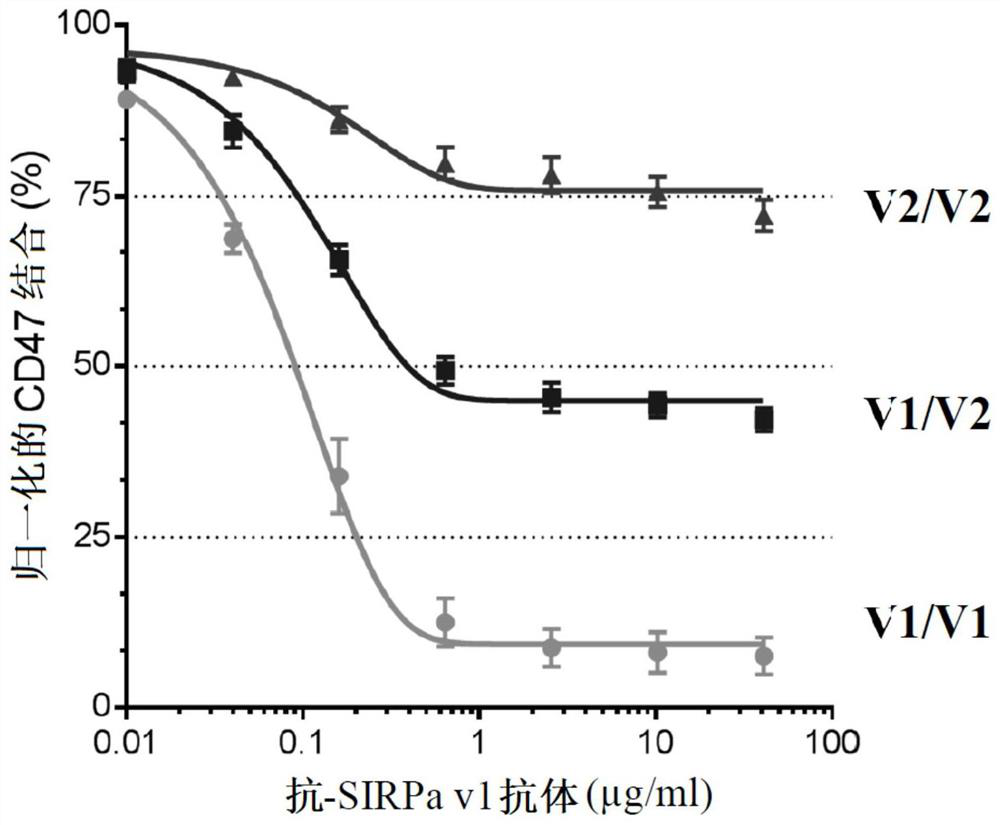
[0302] Example 1: Identification of epitopes capable of producing anti-human SIRPa v1 antibodies
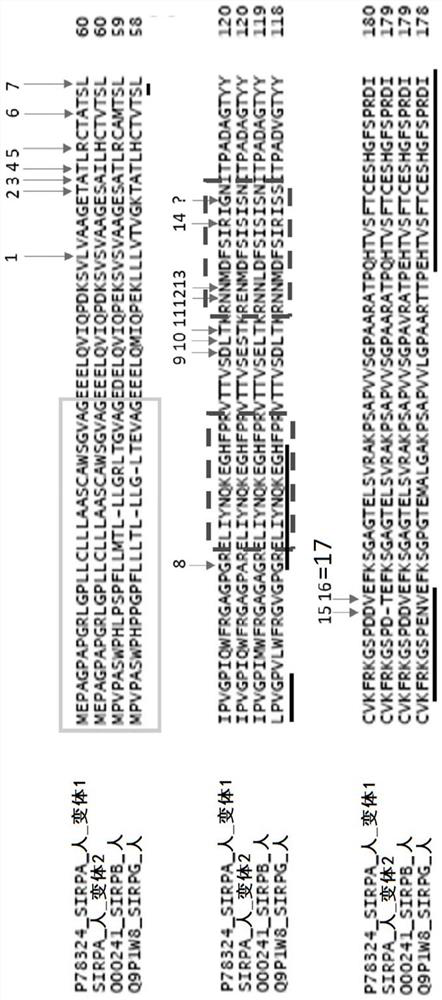
[0303]It was previously described that human SIRPα exhibits a certain degree of polymorphism in domain 1 of IgV that interacts with CD47. This polymorphism is mainly located in exon 3 of the SIRPa gene (SEQ ID NO: 27) (Takenaka et al., 2007). identified 10 distinct sequences / alleles in genome sequences from 37 different donors from different sources, including 2 major alleles: variant 1 (v1) and variant 2 (v2) . Other alleles differed by only 1 or 2 SNPs from the V1 or V2 sequence. Thus, the SIRPa family can be subdivided into two subfamilies: the SIRPa v1 isoform and the SIRPa v2 isoform. These different alleles result in slightly different proteins, but all variants bind the CD47 ligand similarly. In the article by Takenaka et al., the v1 allele frequency was 78% (V1-like was 89%), while the genotype frequency of homozygous V1 / V1-like donors was 65%. 24% of their 37 dono...
Embodiment 2
[0333] Example 2: Comparison of the effect of blockade of the SIRPa-CD47 interaction between mice and humans on immune cross-presentation
[0334] Mouse cross-presentation ( Figure 5 )
[0335] Drugs: An allosteric antagonistic monoclonal antibody targeting mouse SIRPα domain 2 (P84 clone – rat IgG1) was purified from a hybridoma. An orthosteric antagonistic monoclonal antibody targeting mouse SIRPα domain 1 (clone MY-1 – mouse IgG2a) (Garcia et al., 2011) was reengineered to the IgG1 Fc domain of the parental hybridoma. Both anti-SIRPα antibodies blocked signaling through SIRPα in myeloid cells. Isotype control mouse IgGl (clone 3G8) was purified. A surrogate antagonist anti-mouse CD47 monoclonal antibody (MIAP410 clone) was purchased from BioXCell (#BE0283).
[0336] Expression of mouse SIRPa by splenic DC: Native DC were isolated from spleens of naive mice by CD11c-positive magnetic separation, and their CD8α expression was isolated by BD FACS ARIA II cell sorting....
Embodiment 3
[0353] Example 3: Effect of SIRP / CD47 Blockade on Polyclonal Stimulation
[0354] Methods: hPBMC were isolated from the buffy coat of healthy volunteers. CD4 or CD8 T cells were screened by positive selection using AutoMACS (Miltenyi) and plated in 96-well plates (50000 cells / well). Proliferation signals were provided by anti-CD3 / anti-CD28 coated microbeads (Life Technologies) (at a ratio of 1 microbead:1 T cell) over a period of 3 days, or by allogeneic mature dendritic cells generated in vitro ( 5T cells: 1 mDC) were provided over a period of 5 days. Starting from the proliferation assay, antibodies targeting the SIRPa / CD47 and / or SIRPg / CD47 pathways were added at saturating concentrations (10 μg / mL). By incorporation of H during the last 12 hours of culture 3 -thymidine to measure proliferation. Anti-CD47 antibody (commercially available reference number: B6H12), anti-SIRPa antibody (HEFLB, as described in patent WO2017178653).
[0355] Results: To study the immunosu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com