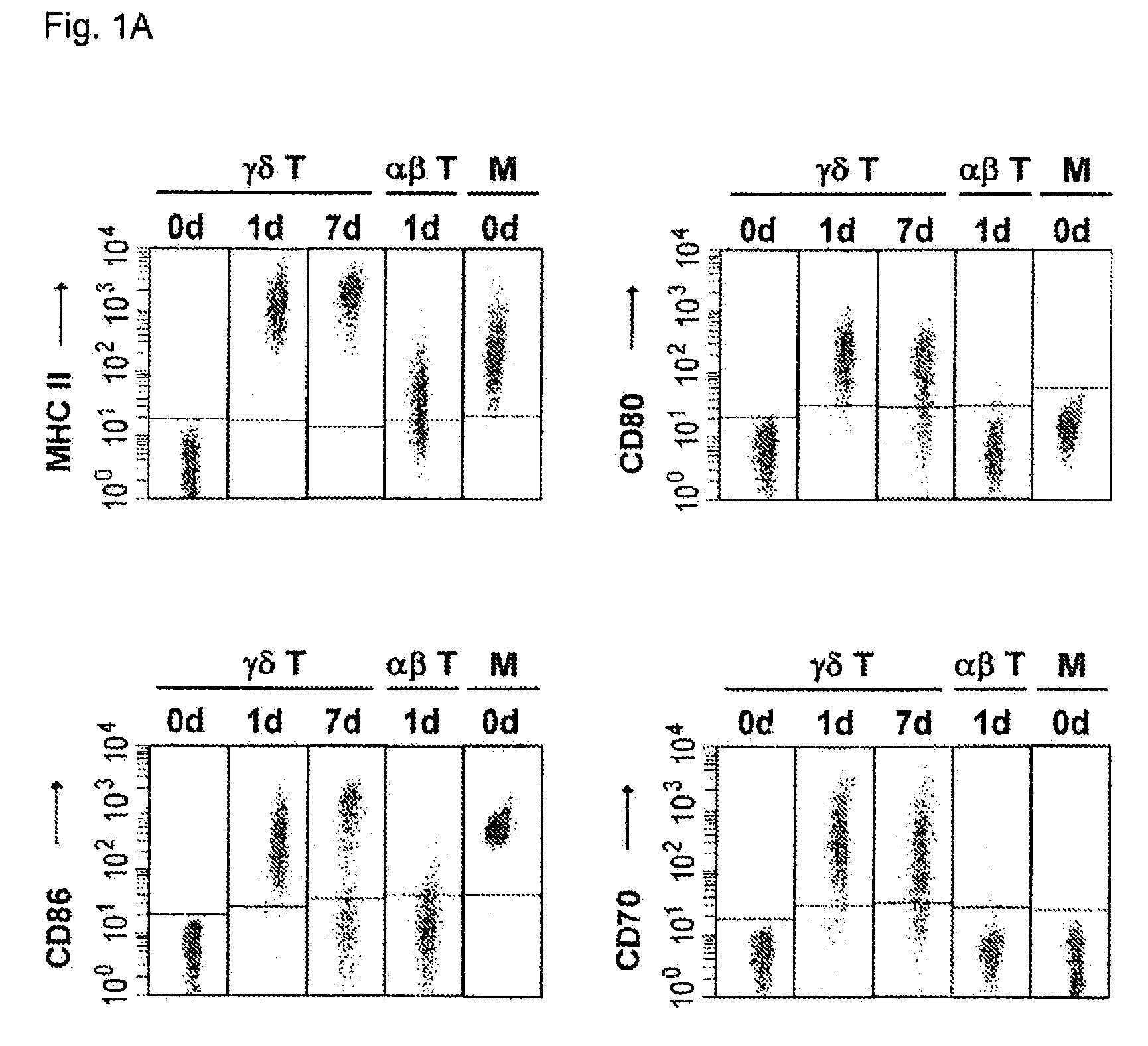
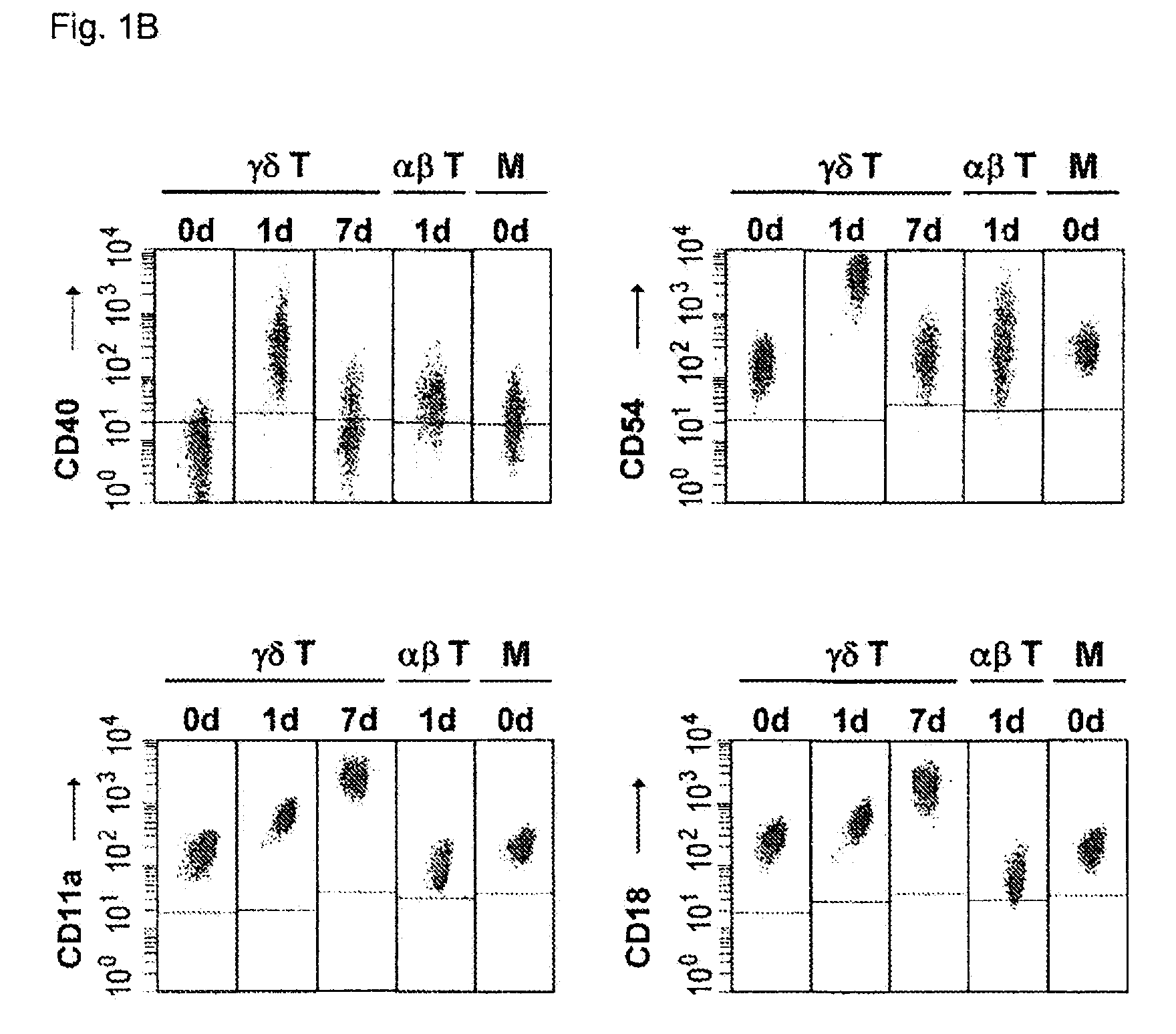
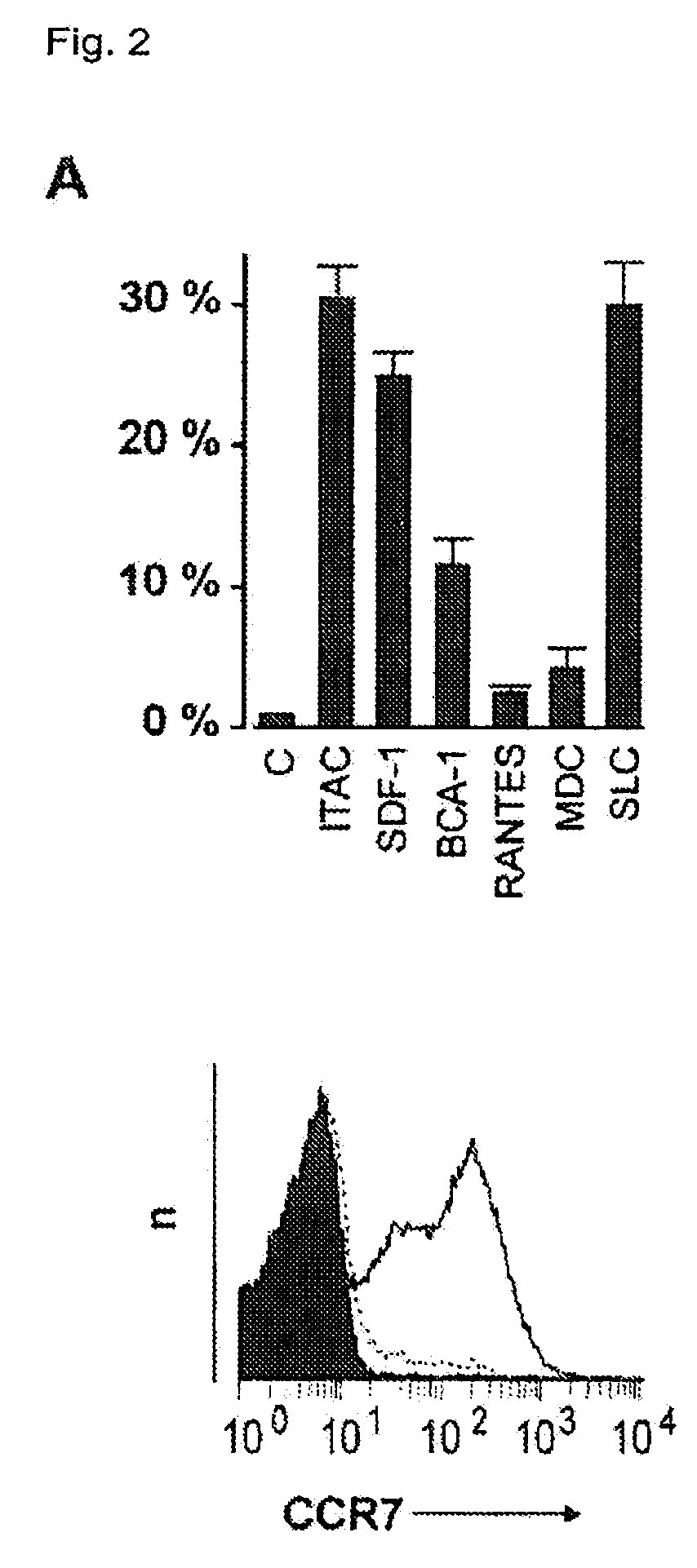
Preparation of antigen-presenting human γδ T cells and use in immunotherapy
a technology which is applied in the field of preparation of human t cells and immunotherapy, can solve the problems of not being further defined, poorly immunogenic, and not supporting the role of t cells in antigen presentation, and achieves the effects of promoting antigen presentation, facilitating purification, and maintaining efficient antigen-presenting functions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Human γδ T-APCs Efficiently Cross-Present Soluble Proteins to CD8+αβ T Cells
[0148]First, we examined the ability of γδ T-APCs to induce αβ T cell proliferation in response to the highly complex protein mixture M. tuberculosis purified protein derivative (PPD). γδ T-APCs or monocyte-derived DCs were loaded with PPD, washed and then co-cultured with autologous, 5-(and 6-) carboxyfluorescein diacetate succinimidyl ester (CFSE)-labeled responder cells. Using bulk CD3+ T cells as responder cells, both CD8+ T cells and CD4+ T cells showed strong proliferation responses, as assessed by reduction in CFSE signals (FIG. 18 A). Similar antigen-dependent responses were obtained with purified naïve CD8+αβ T cells as responder cells. We concluded that γδ T-APCs compared well with DCs in the induction of CD8+αβ T cell responses to complex mycobacteria-derived protein antigens.
[0149]To confirm these initial findings in support for cross-presentation by γδ T-APCs, we turned to an experimental model ...
example 2
Antigen Cross-Presentation by γδ T-APCs Involves Proteasome Activity and de Novo Synthesized MHC I Molecules
[0151]The efficiency of antigen presentation to CD8+αβ T cells correlates with the rate of de novo synthesis of MHC I and transport of peptide-MHC I complexes from the MHC I peptide loading compartment (endoplasmic reticulum) to the cell surface (Cox et al., 1990). The classical MHC I pathway of peptide presentation involves antigen degradation by the proteasome in the cytoplasm, followed by the transporter associated with antigen processing (TAP)-dependent transport of proteolytic products across the endoplasmic reticulum membrane and loading of peptides onto MHC I molecules (Yewdell et al., 2005; Cresswell et al., 2005; Rock et al., 2005; Villadangos et al., 2007). Alternative, TAP- and proteasome-independent pathways have been proposed, including lysosomal (as opposed to cytoplasmic) degradation of internalized antigen followed by peptide loading onto recycling or cell surf...
example 3
The Immunoproteasome in γδ T-APCs Prevents Induction of Melp26-35-specific CD8+αβ T Cell Responses
[0154]To test a potential function in anti-tumor immunity, we next studied the ability of γδ T-APCs to cross-present the melanocyte / melanoma-differentiation antigen Melan-A (MART-1), which contains the immunodominant peptide Melp26-35 recognized by HLA-A2-restricted CD8+αβ T cells (Romero et al., 2002). Melp26-35 specific CD8+αβ T cells are readily detected in both melanoma patients and healthy individuals (Pittet et al., 1999), thus allowing us to study Melan-A cross-presentation by γδ T-APCs with blood cells from healthy volunteers. Of note, Melan-A-pretreated γδ T-APCs and DCs both failed to induce IFN-γ production in HLA-A2-restricted, Melp26-35-specific responder cell clones (FIG. 21 A, additional data not shown). This failure was not due to problems with antigen presentation per se or due to a weak responsiveness by the responder clone since Melp26-35-pulsed γδ T-APCs and DCs indu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentrations | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com