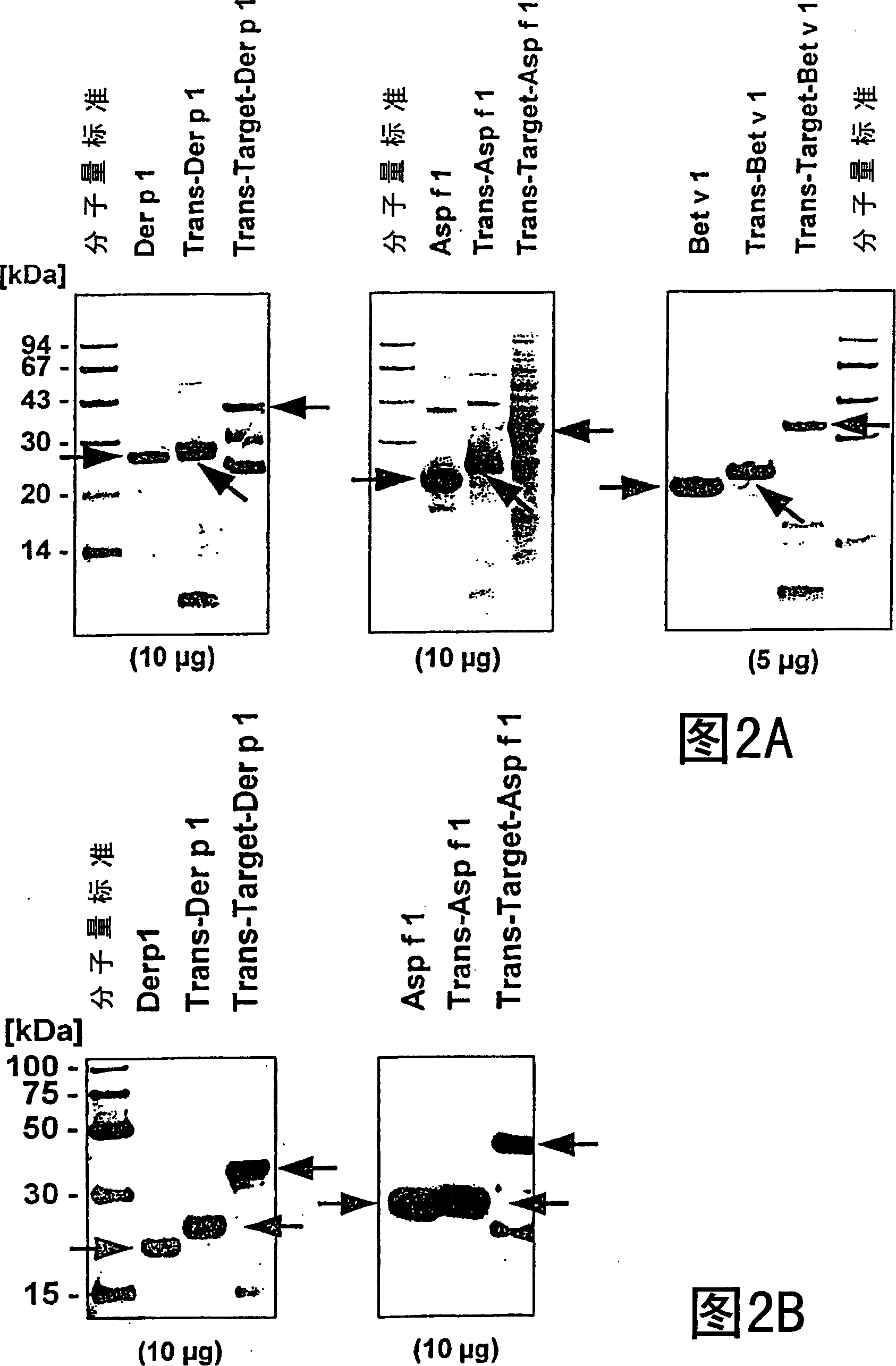
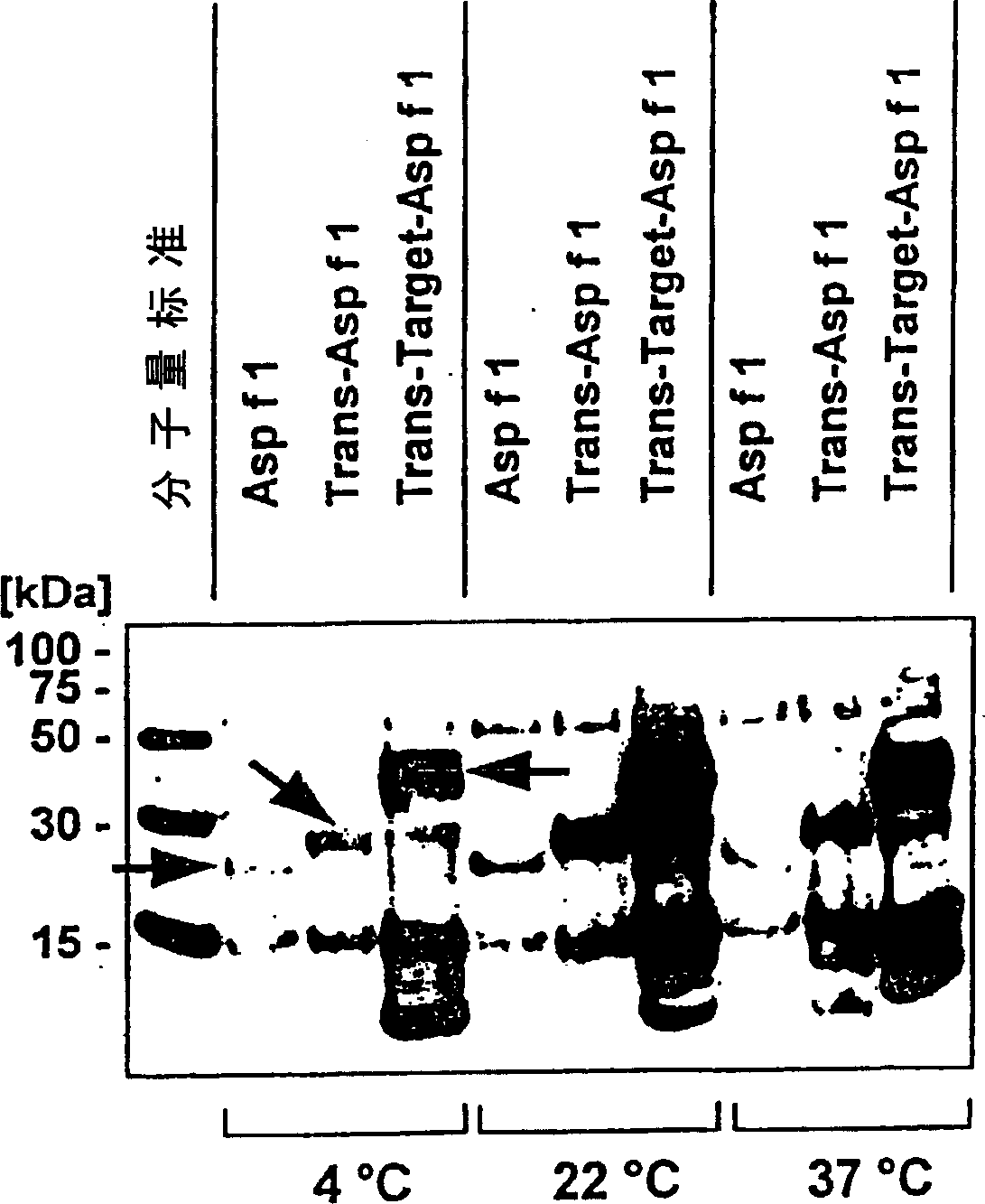
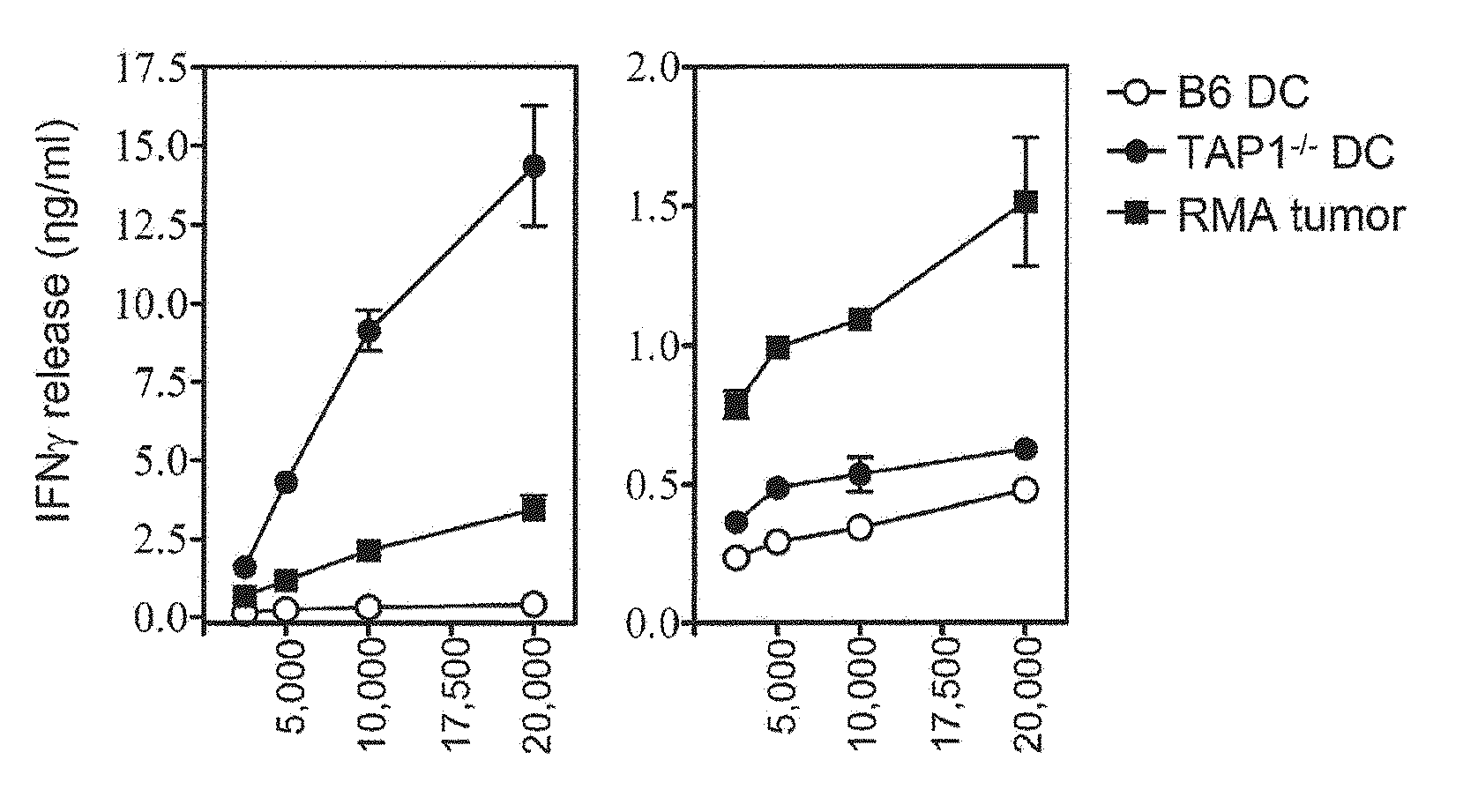
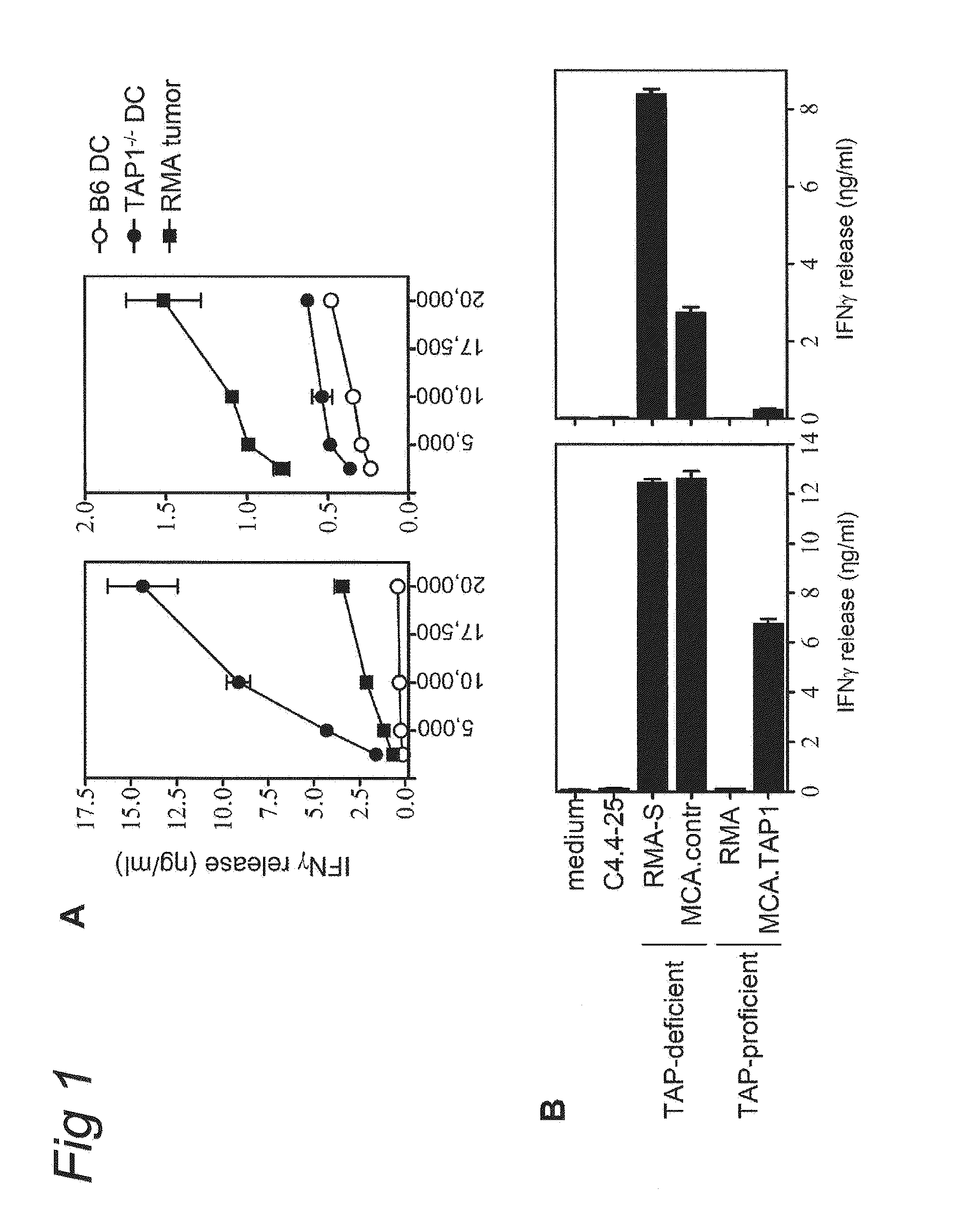
Patents
Literature
43 results about "Antigen processing" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
'Antigen processing or cytosolic' pathway is an immunological process that prepares antigens for presentation to special cells of the immune system called T lymphocytes. It is considered to be a stage of antigen presentation pathways. This process involves two distinct pathways for processing of antigens from an organism's own (self) proteins or intracellular pathogens (e.g. viruses), or from phagocytosed pathogens (e.g. bacteria); subsequent presentation of these antigens on class I or class II major histocompatibility complex (MHC) molecules is dependent on which pathway is used. Both MHC class I and II are required to bind antigen before they are stably expressed on a cell surface. MHC I antigen presentation typically (considering cross-presentation) involves the endogenous pathway of antigen processing, and MHC II antigen presentation involves the exogenous pathway of antigen processing. Cross-presentation involves parts of the exogenous and the endogenous pathways but ultimately involves the latter portion of the endogenous pathway (e.g. proteolysis of antigens for binding to MHC I molecules).
Herpes virus vectors for dendritic cells
InactiveUS6641817B1Efficient infectionLow toxicityBiocideGenetic material ingredientsDiseaseInfected cell
An attenuated herpes virus capable of efficiently infecting a dendritic cell without preventing antigen processing occurring within the infected cell. The attenuated herpes virus and dentrictic cells infected with the virus are useful in immunotherapeutic methods of treating disease.
Owner:BIOVEX LTD
Improved HLA epitope prediction
PendingUS20190346442A1Microbiological testing/measurementMass spectrometric analysisT cellHuman health
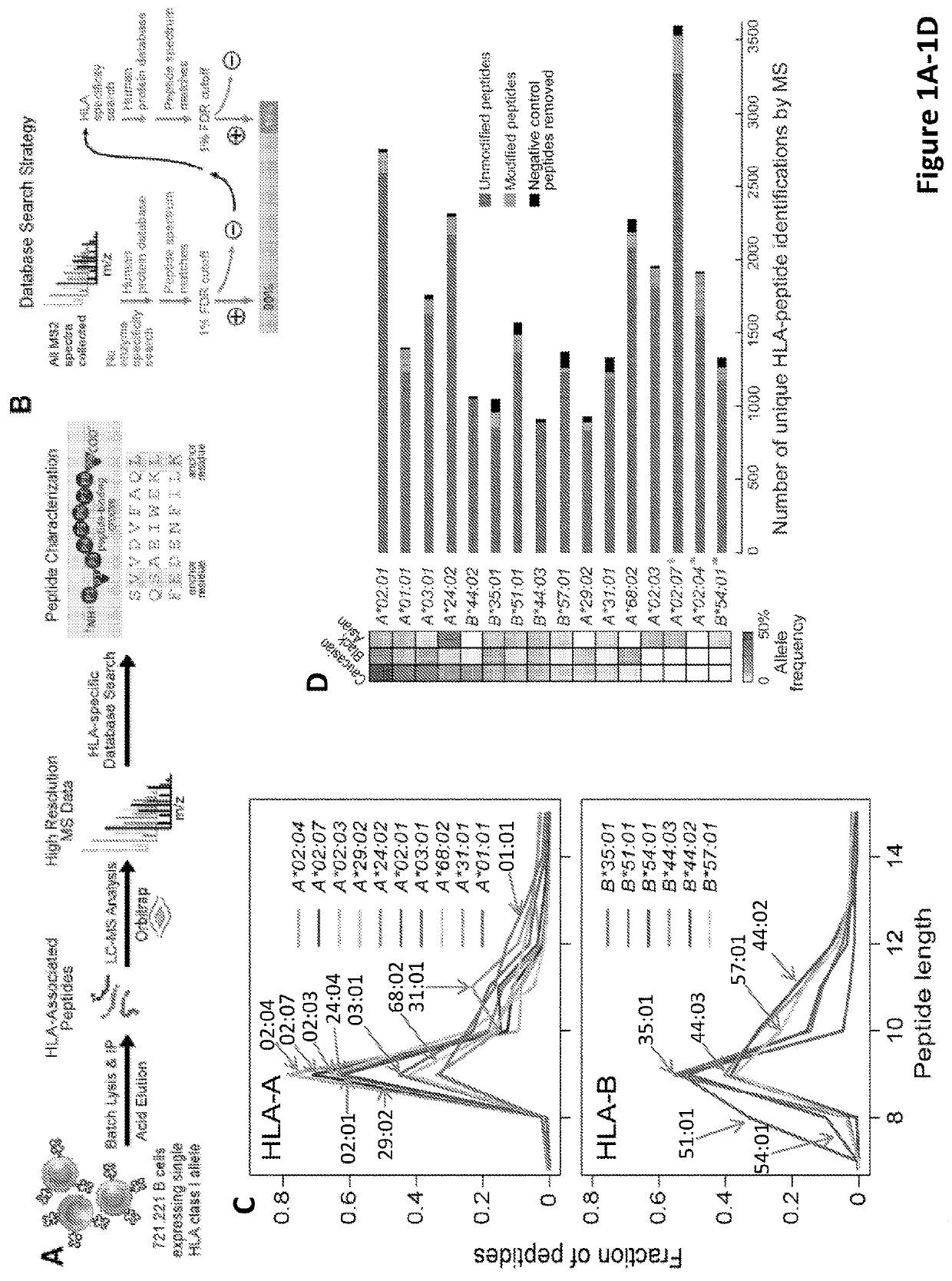
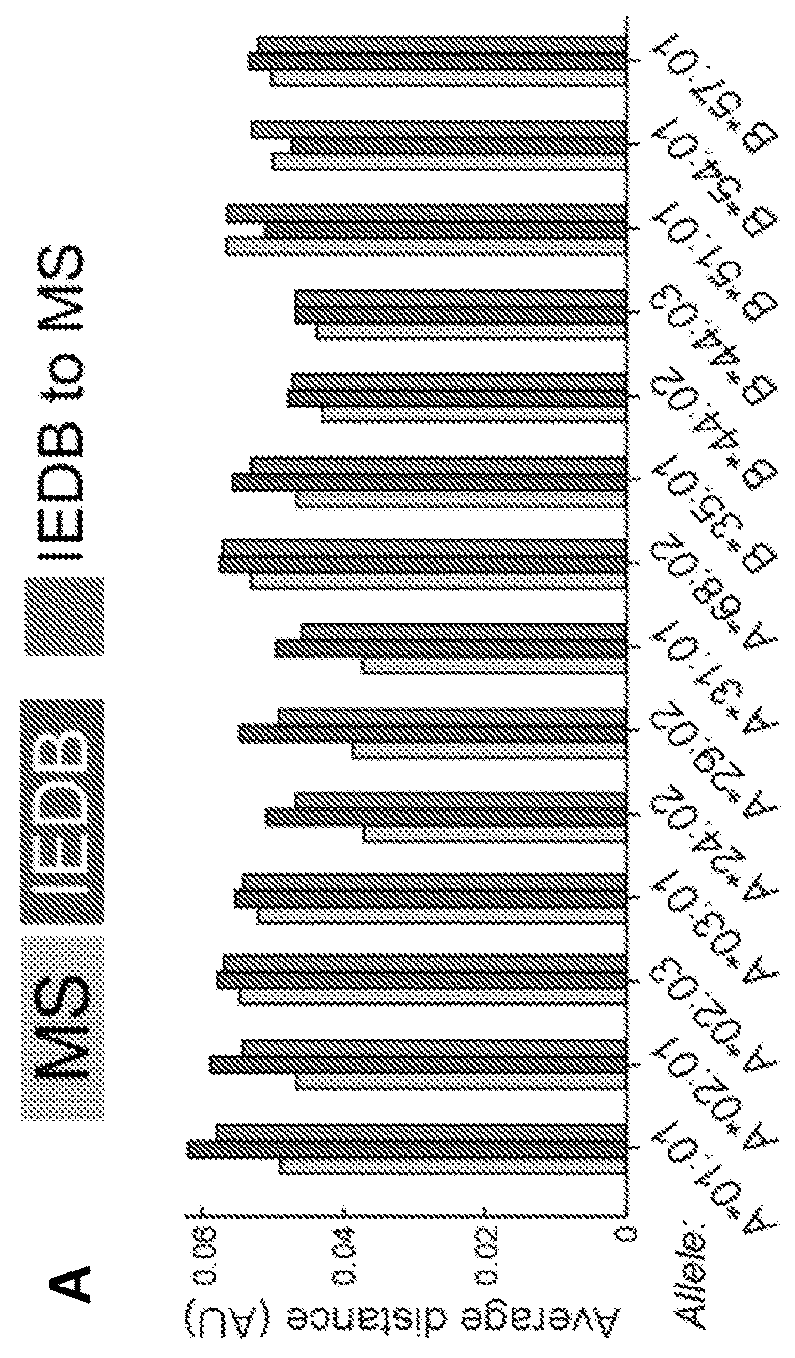
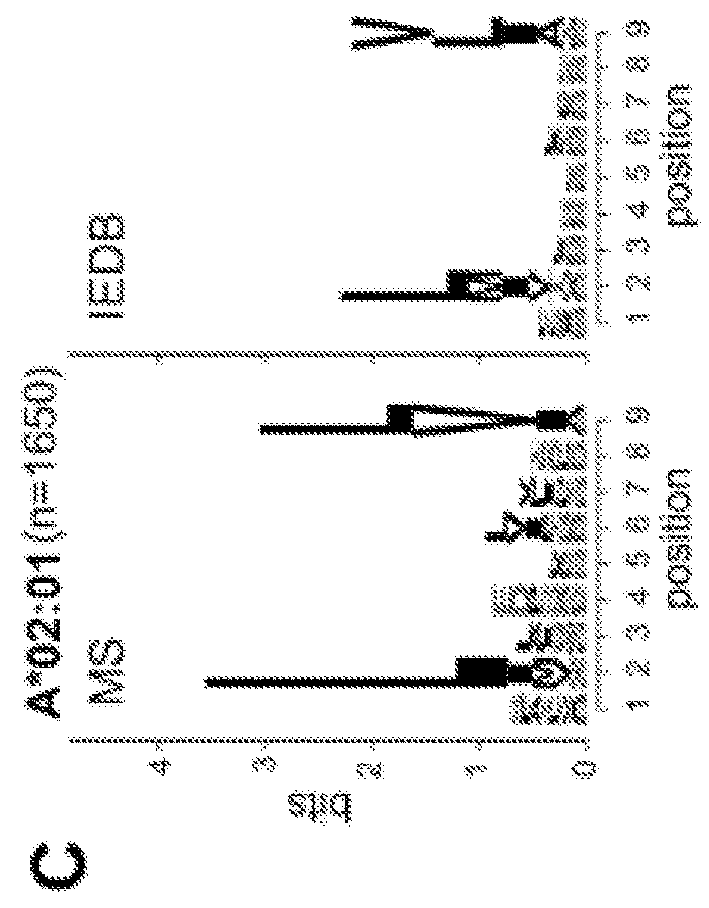
Adaptive immune responses rely on the ability of cytotoxic T cells to identify and eliminate cells displaying disease-specific antigens on human leukocyte antigen (HLA) class I molecules. Investigations into antigen processing and display have immense implications in human health, disease and therapy. To extend understanding of the rules governing antigen processing and presentation, immunopurified peptides from B cells, each expressing a single HLA class I allele, were profiled using accurate mass, high-resolution liquid chromatography-mass spectrometry (LC-MS / MS). A resource dataset containing thousands of peptides bound to 28 distinct class I HLA-A, -B, and -C alleles was generated by implementing a novel allele-specific database search strategy. Applicants discovered new binding motifs, established the role of gene expression in peptide presentation and improved prediction of HLA-peptide binding by using these data to train machine-learning models. These streamlined experimental and analytic workflows enable direct identification and analysis of endogenously processed and presented antigens.
Owner:THE BROAD INST INC +4
Modular antigen transporter molecules (MAT molecules) for modulating immune reactions, associated constructs, methods and uses
ActiveCN1738901AEffective specific immune responseEffective immune responseAntibody mimetics/scaffoldsAntipyreticAntigenAntigen processing
By adding the novel developed modular antigen transporter molecules (MAT molecules) and other instruments related thereto, the immune system of an individual can be effectively modulated in a targeted manner. The MAT molecules contain a translocation module which transports the MAT molecule inside the cell, an active intracellular targeting molecule resulting in intracellular transport of the MAT molecule to the organelles of the cell which are responsible for antigen processing and an antigen module which determines the specificity of the immune response modulated by the MAT molecule. Optionally, other modules such as tag modules or spacer modules can be contained in the MAT molecule. The invention comprises the inventive MAT molecules, antibodies which are produced using said MAT molecules and therapeutic agents and diagnostic reagents which are produced with MAT molecules. The invention also comprises applications containing said MAT molecules, antibodies, therapeutic agents and diagnostic reagents.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Use of a varicellovirus tap-inhibitor for the induction of tumor-or virus-specific immunity against teipp
InactiveUS20100092435A1Reduce transportationEffective blockingBiocidePeptide/protein ingredientsAntigenAbnormal tissue growth
The present invention provides a novel approach to the modulation of the immune response, directing it towards specific antigens, away from antigens against which no response is desired. The invention is based on the use of viral immune evasion proteins, such as UL49.5, which block antigen presentation to CD8+ T cells. The viral immune evasion proteins are used for: 1) the induction of tumor-specific or virus-specific immunity in cases where a conventional immune response is absent due to antigen processing defects; 2) the induction of empty MHC class I molecules at the cell surface that can be loaded with peptides of a desired specificity; 3) the inhibition of unwanted immune responses against transplanted tissues or organs, e.g. against islets of Langerhans in type 1 diabetes or allogeneic stem cells, or against self antigens in the case of autoimmunity.
Owner:PUBLIEKRECHTELIJKE RECHTSPERSOON ACADEMISCH ZIEKENHUIS LEIDEN H O D N LEIDS UNIVIR MEDISCH CENT
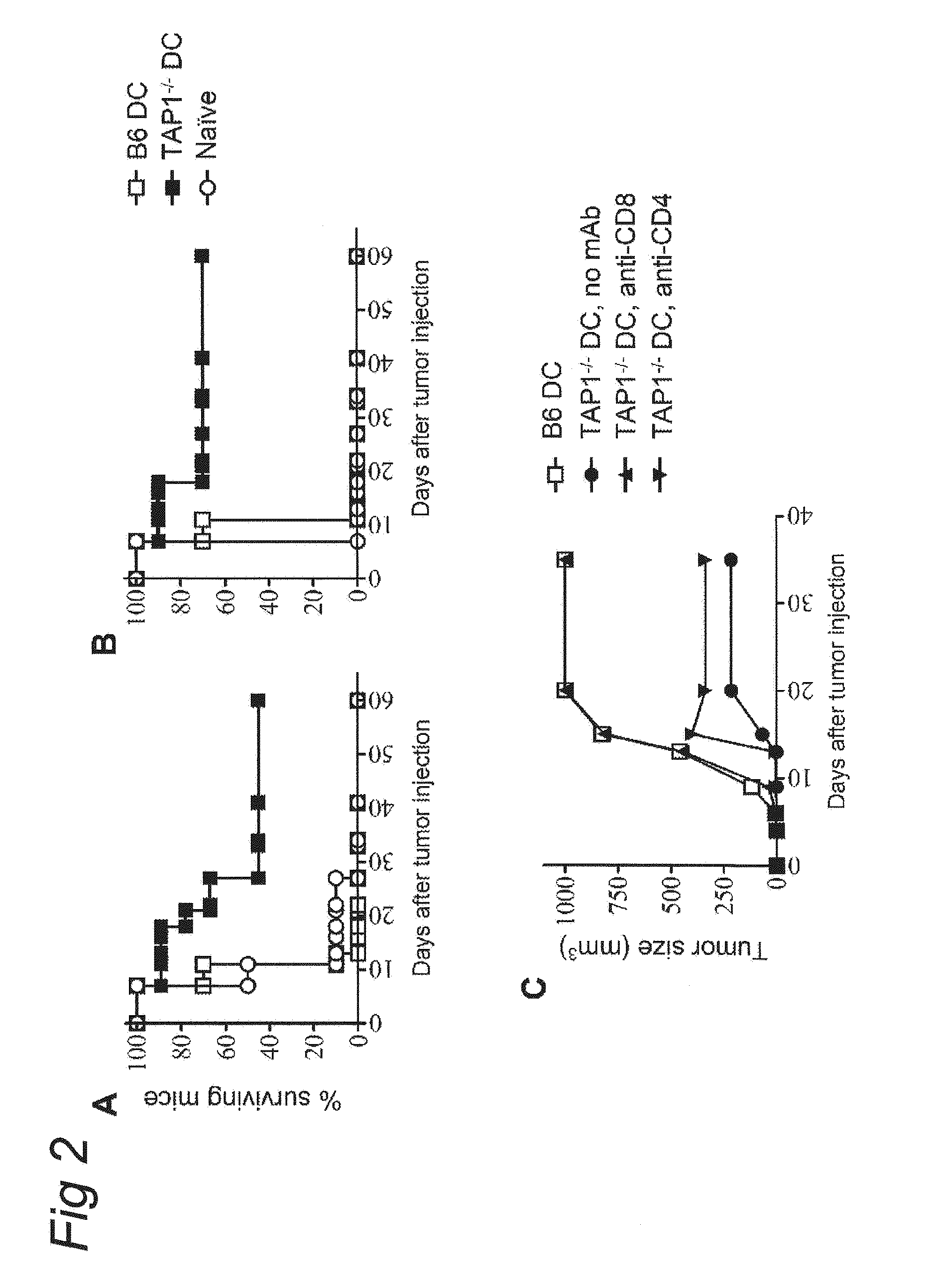
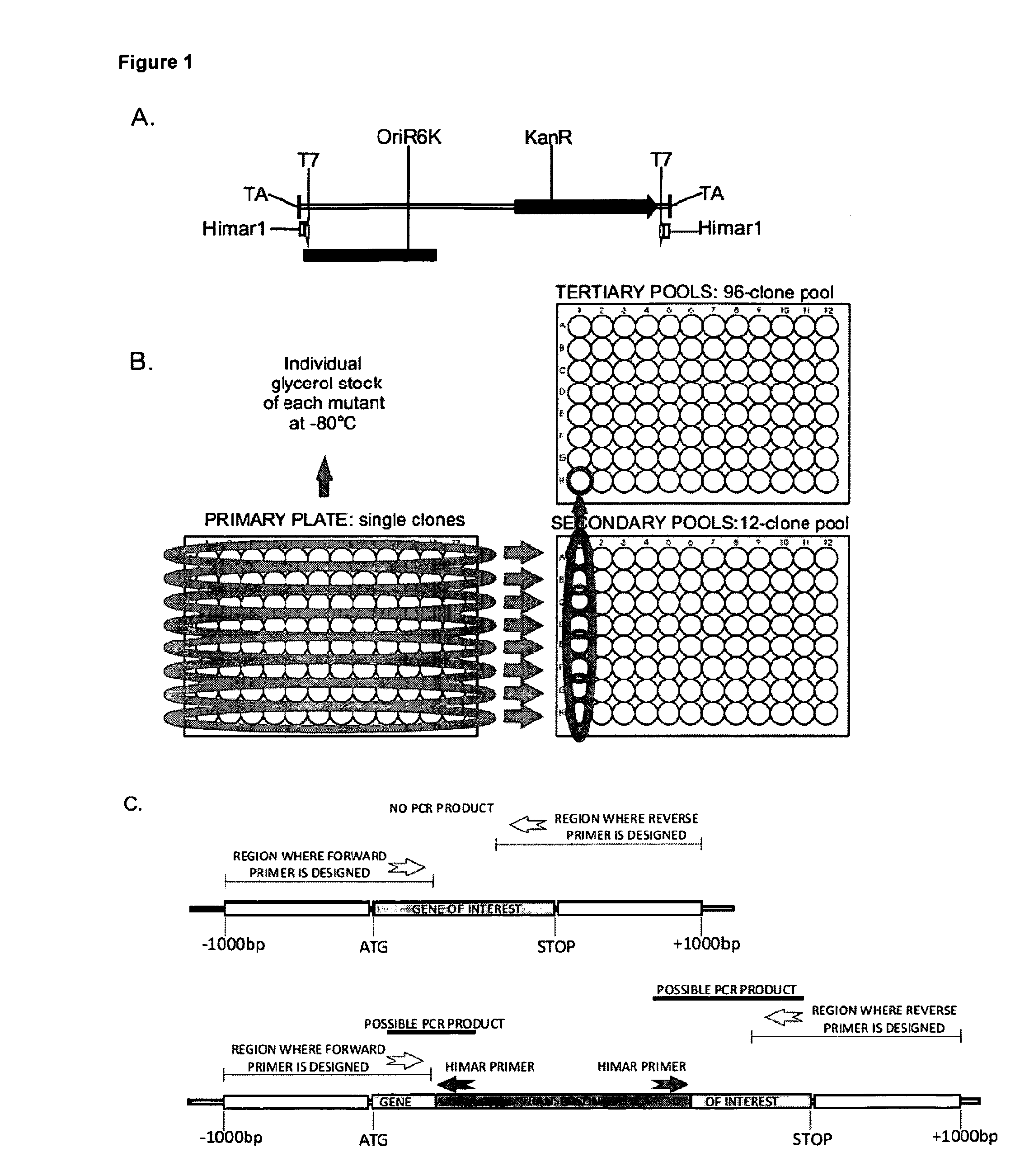
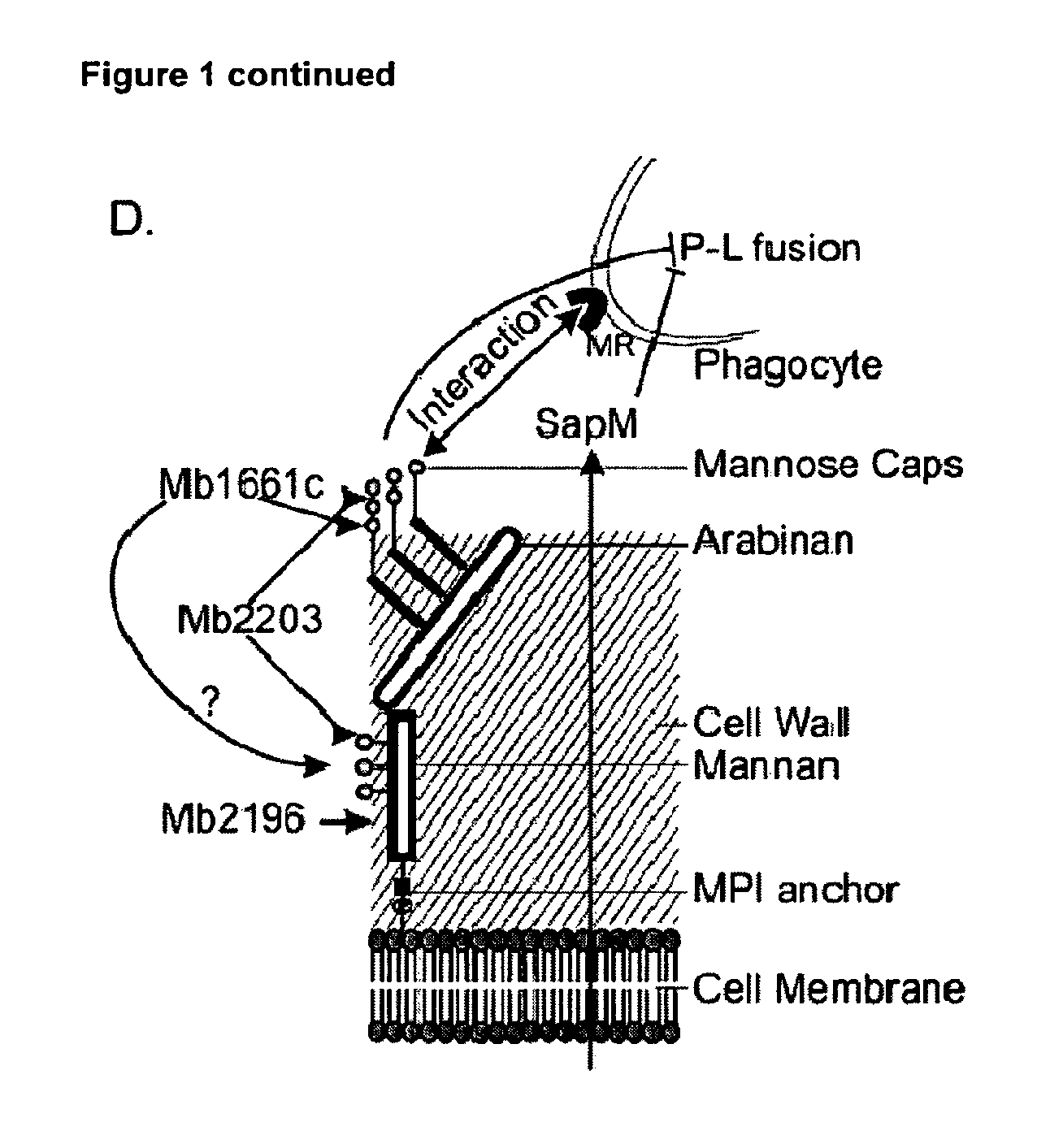
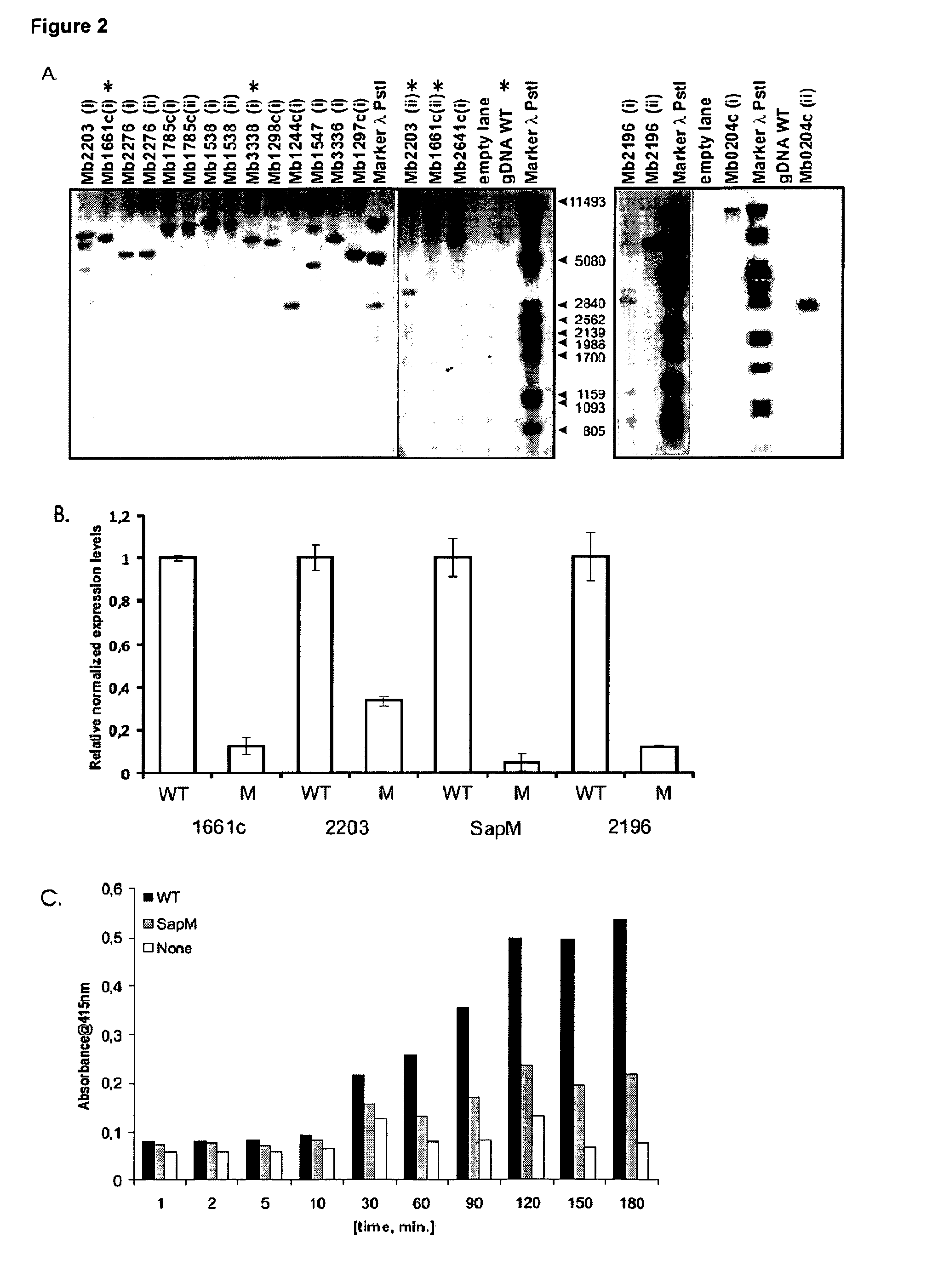
Mycobacterium mutants for vaccines with improved protective efficacy
Tuberculosis (TB) is a major health problem and currently, the only licensed TB vaccine is Mycobacterium bovis Bacille Calmette-Guerin (M. bovis BCG). In the present invention, mutation of mycobacterial components reportedly involved in phagosome maturation inhibition was evaluated for vaccine purposes, as such mutations should result in better vaccine antigen processing and presentation. Thus, BCG mutants in genes coding for ManLAM capping a-1,2-mannosyltransferases and the PI3P phosphatase SapM were evaluated as TB vaccines in a stringent mouse model. Vaccination with both ManLAM capping mutants and the SapM mutant resulted in significantly longer survival as compared to non-vaccinated mice, whereas vaccination with the parental BCG did not. Moreover, mice vaccinated with the SapM mutant survived significantly longer than mice vaccinated with the parental BCG.; The mutant BCG strains showed unaltered phagocytosis, replication, lysosome colocalization and oxidant activity in macrophages and similarly induced autophagy in the latter. Additionally, replication and granuloma formation in mice was unaffected, indicating BCG-equivalent safety of these vaccines.
Owner:VLAAMS INTERUNIVERSITAIR INST VOOR BIOTECHNOLOGIE VZW +2
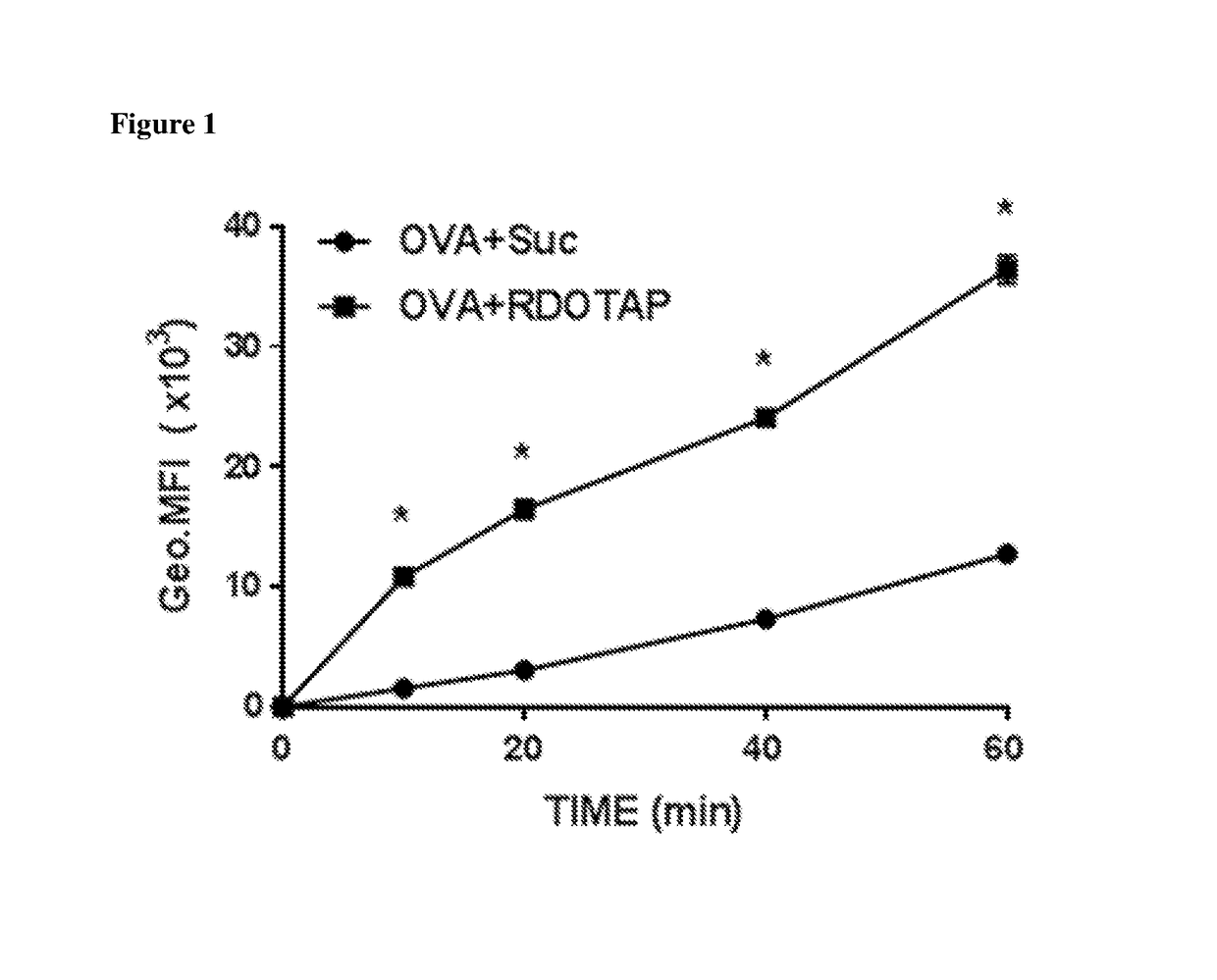
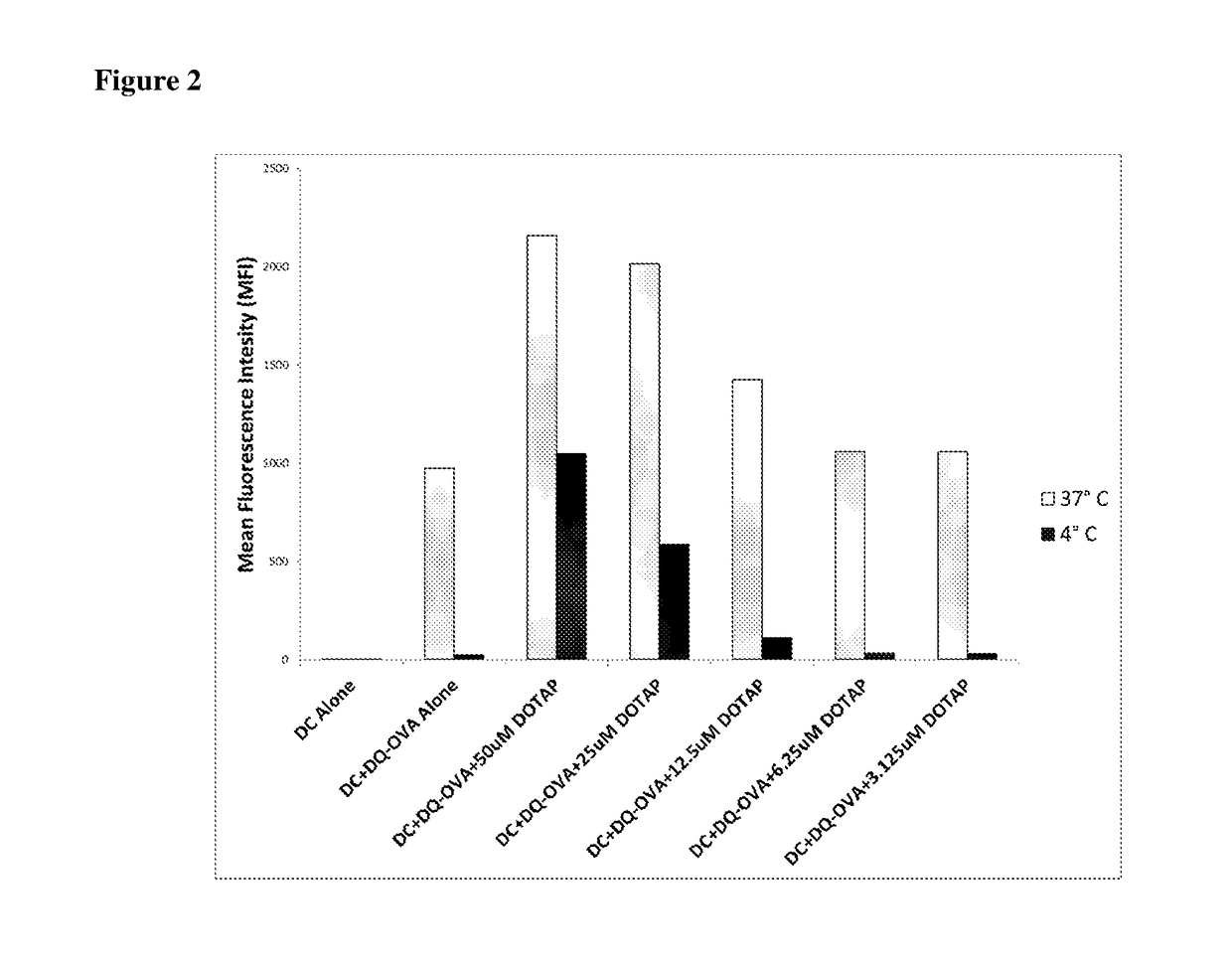
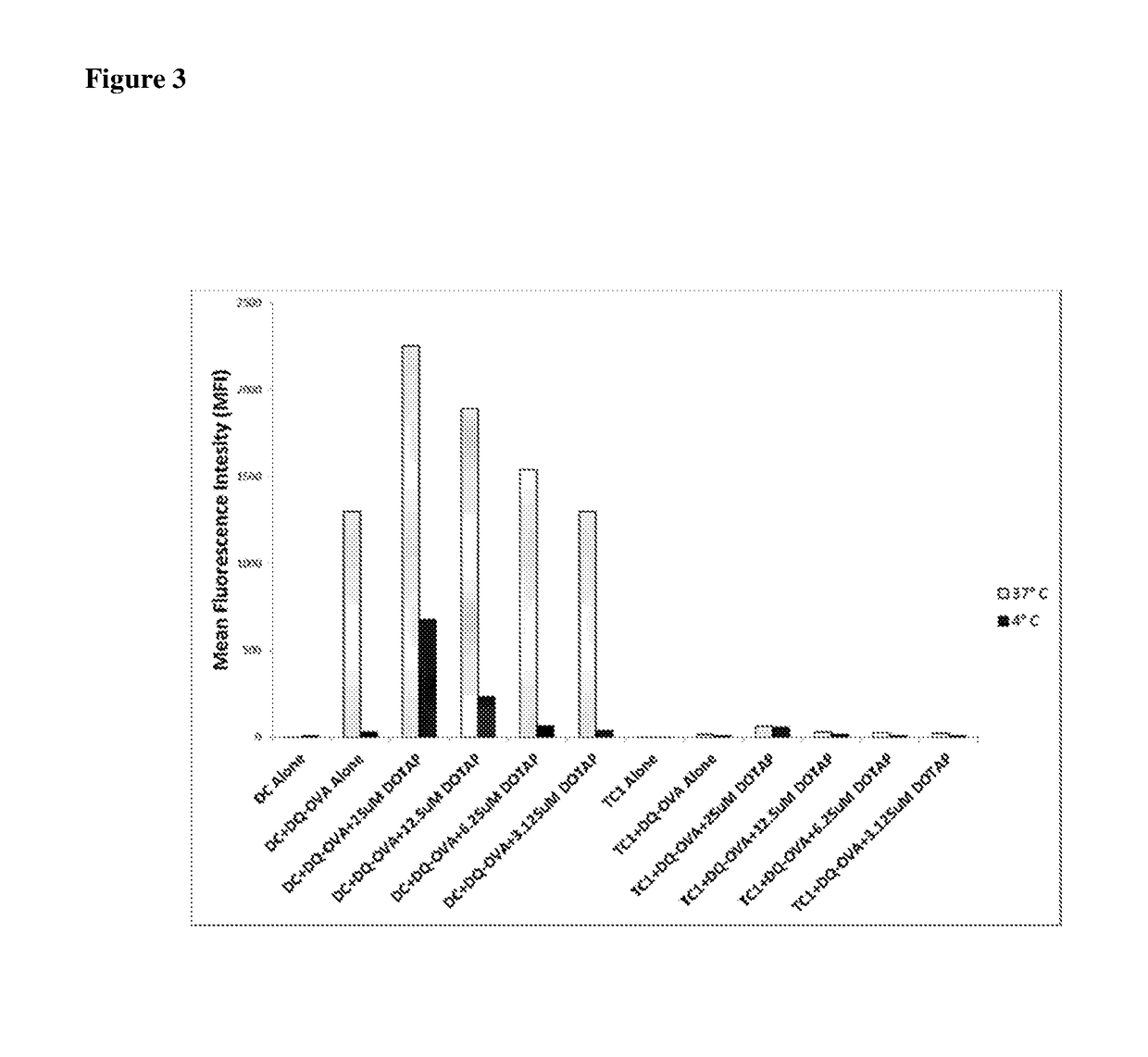
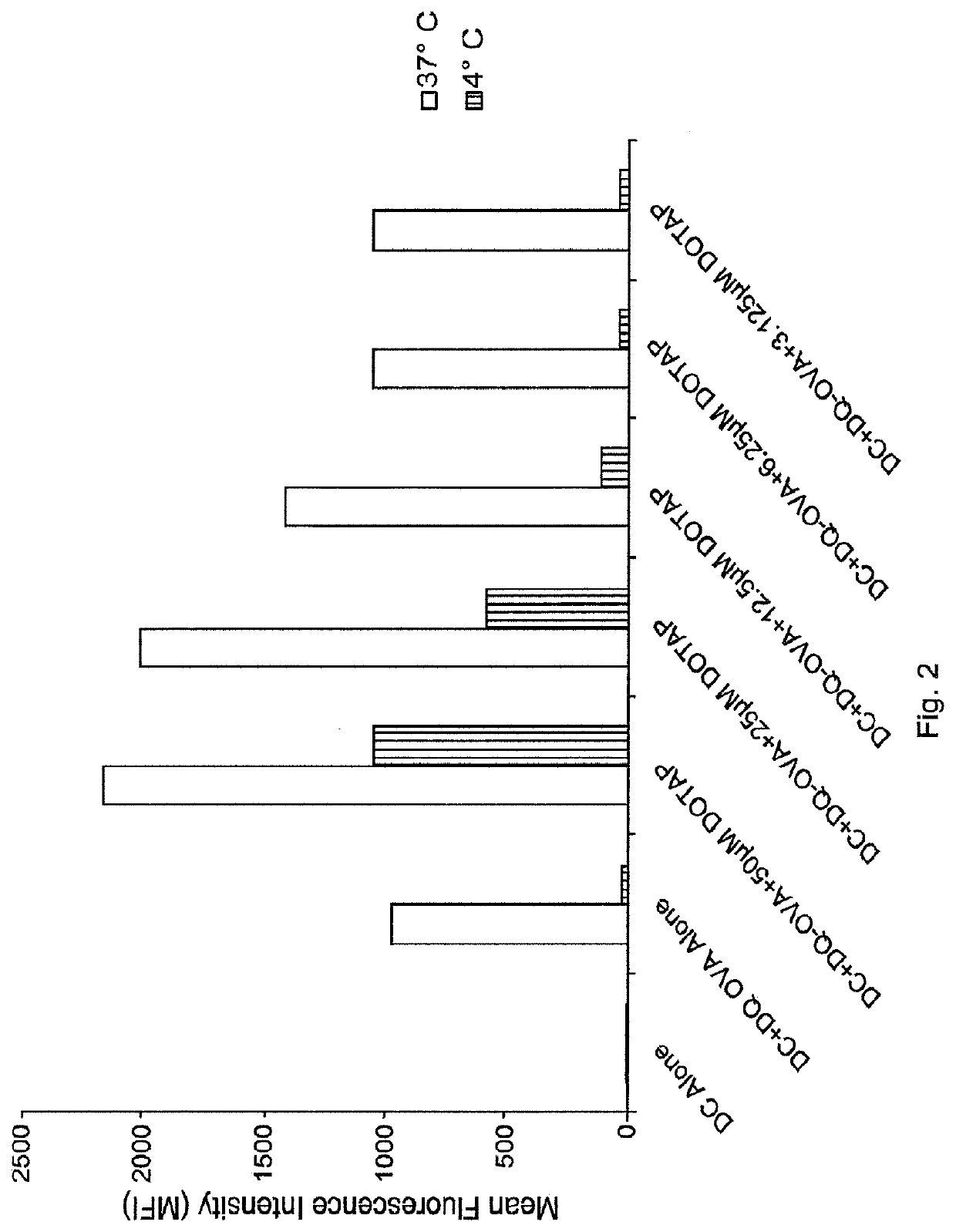
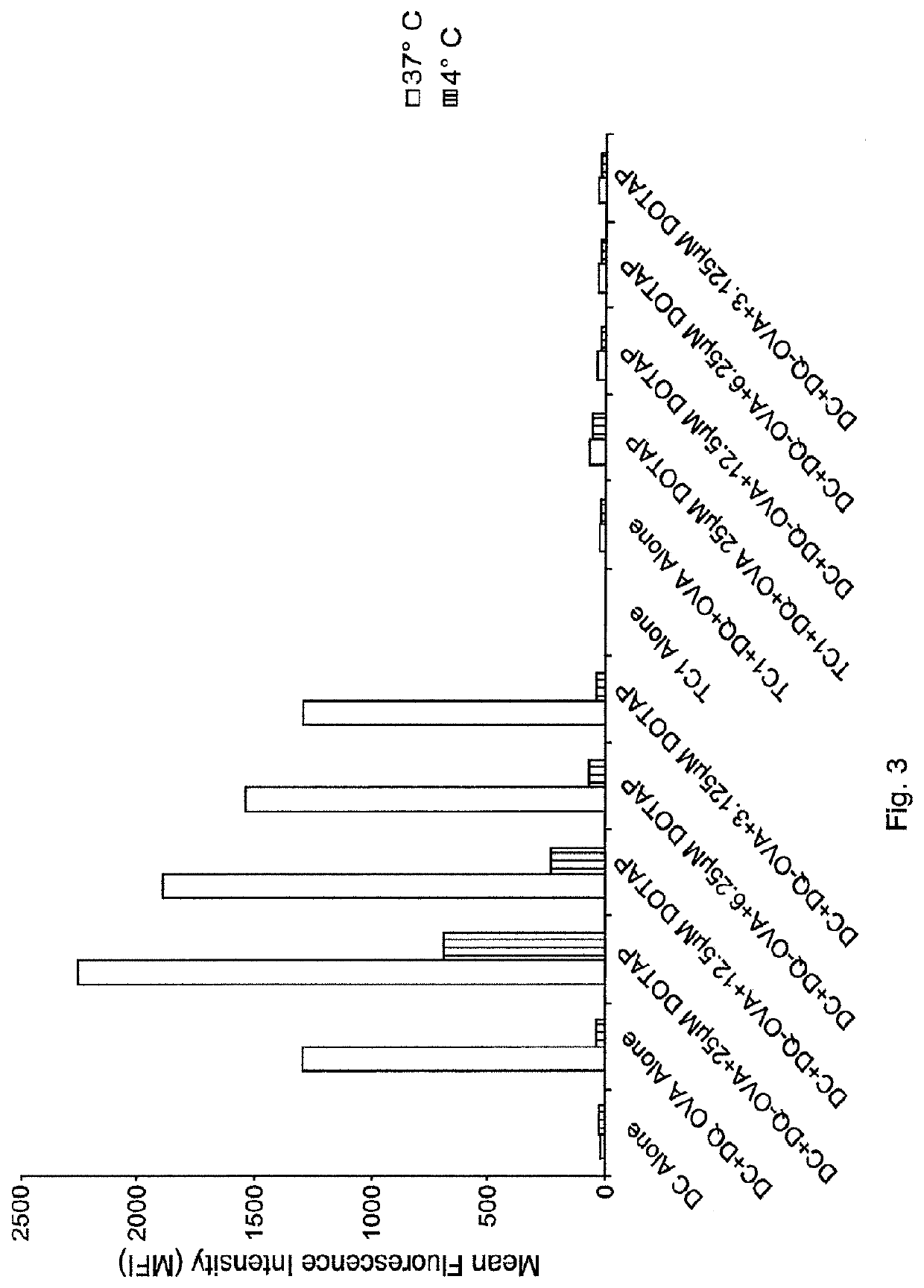
Lipids as synthetic vectors to enhance antigen processing and presentation ex-vivo in dendritic cell therapy
ActiveUS20180353599A1Improve the level ofLow level of TregViral antigen ingredientsMammal material medical ingredientsTumor targetRegulatory T cell
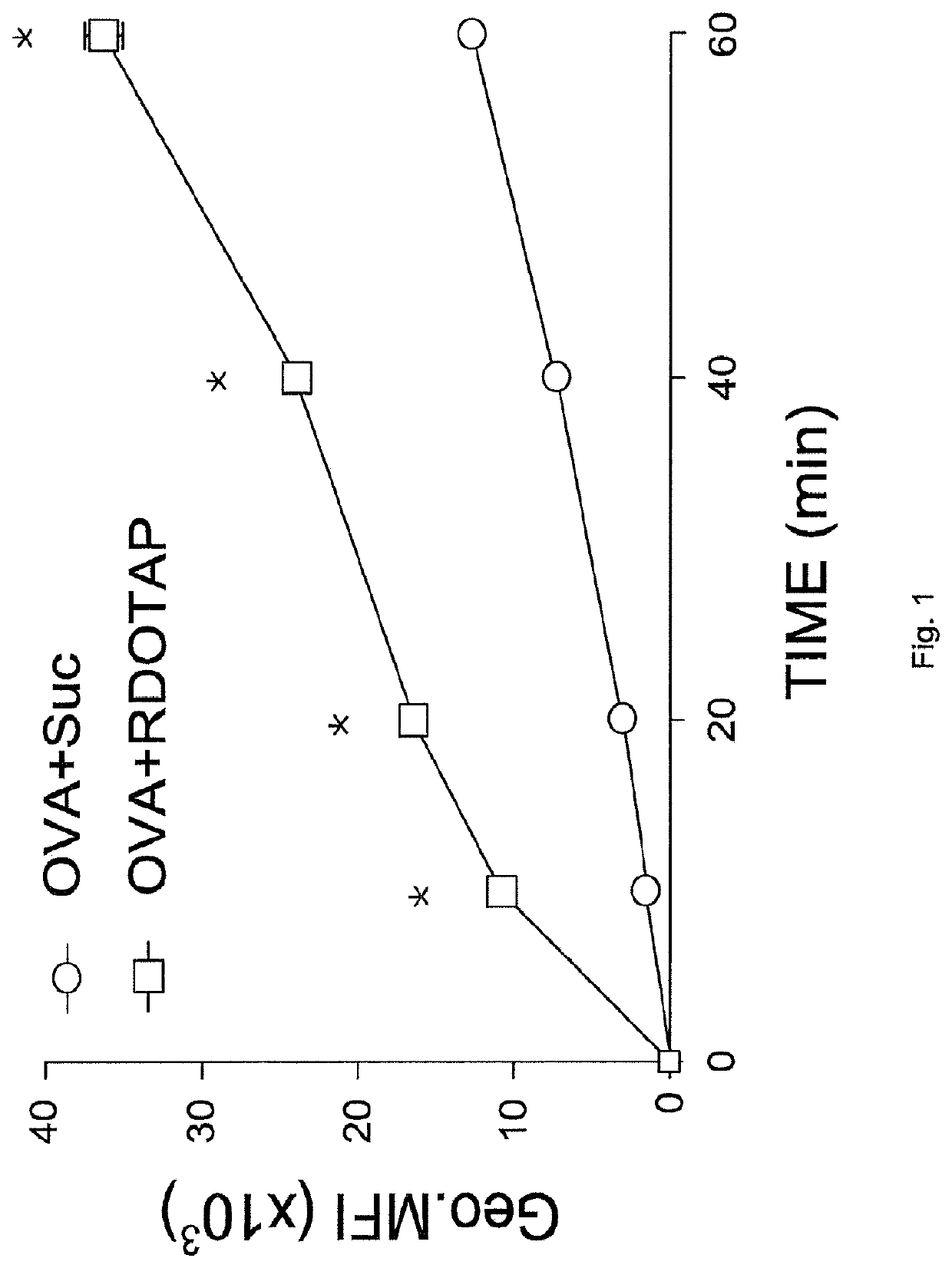
The invention covers the use of certain classes of lipids including cationic lipids in ex-vivo dendritic cell therapies. The cationic lipids enhance antigen uptake, processing and presentation of the processed antigens by dendritic cells to CD8+ and CD4+ T-cells via the MHC classes I and II presentation pathways respectively. Antigen uptake via cationic lipid by dendritic cells result in significant lowering of the population of the immune suppressive regulatory T cells in the tumors and a significant increase of the tumor targeting cytotoxic T-cells. Loss of regulatory T cells and increase of tumor specific cytotoxic cells are conducive to effective elimination of the tumors.
Owner:PDS BIOTECH
Genetic engineering subunit oral vaccine strain for preventing porcine epidemic diarrhea as well as construction method and application thereof
InactiveCN107961373AEco-friendly designGood probiotic propertiesBacteriaMicroorganism based processesDendritic cellVaccine antigen
The invention discloses a genetic engineering subunit oral vaccine strain for preventing porcine epidemic diarrhea as well as a construction method and an application thereof. Autochthonous lactic acid bacteria strains which are separated from intestinal tract of pigs are used as transferring active carriers of vaccine antigens, PEDV S protein neutralizing antigen core epitope domain COE is selected as an immunogen, and at the same time transport speed of the vaccine antigen is effectively improved, immune response time of bodies is shortened, an antigen transporting cell M cell targeting peptide Col and dendritic cell targeting peptide DCpep for antigen processing and presentation is introduced, a constitutive expression carrier is used for constructing a recombined lactic acid bacteria preparation for expressing the PEDV S protein neutralizing antigen core epitope domain COE and a M cell targeting peptide Col and a dendritic cell targeting peptide DCpep, so that the vaccine antigen secretes along with breeding of genetic engineering bacterial strains, and disadvantages of induction type recombined lactic acid bacteria are avoided. An effective technical means is provided for developing the genetic engineering subunit oral vaccine strain for preventing porcine epidemic diarrhea is provided.
Owner:NORTHEAST AGRICULTURAL UNIVERSITY
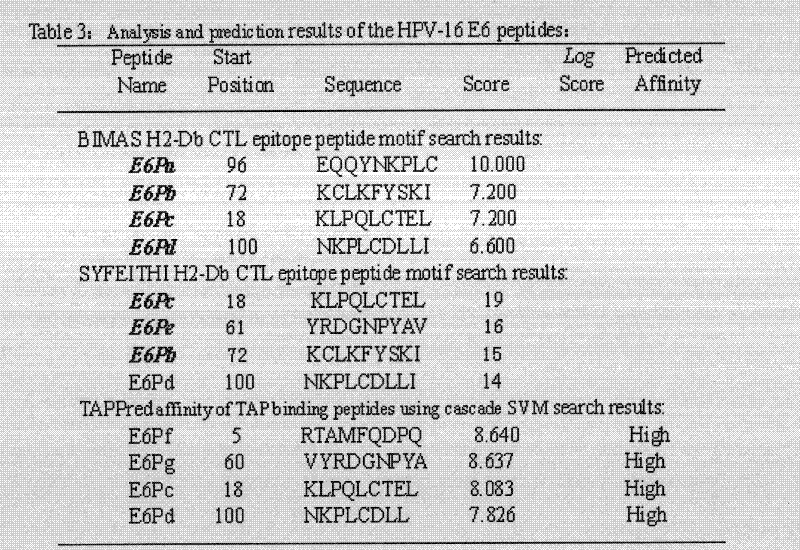
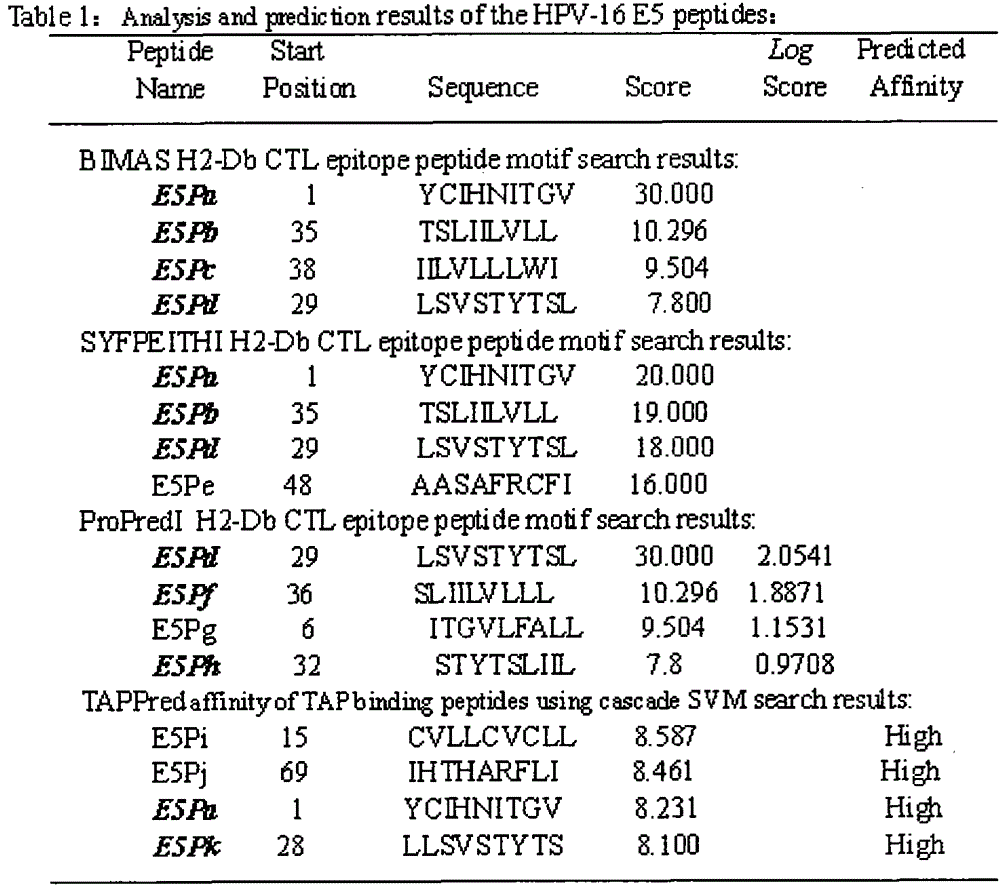
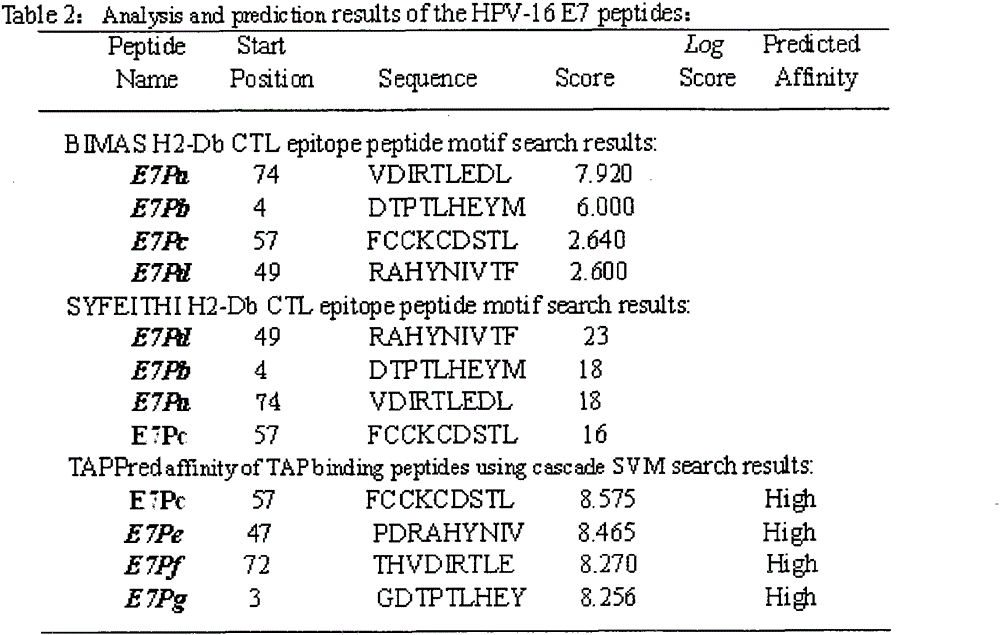
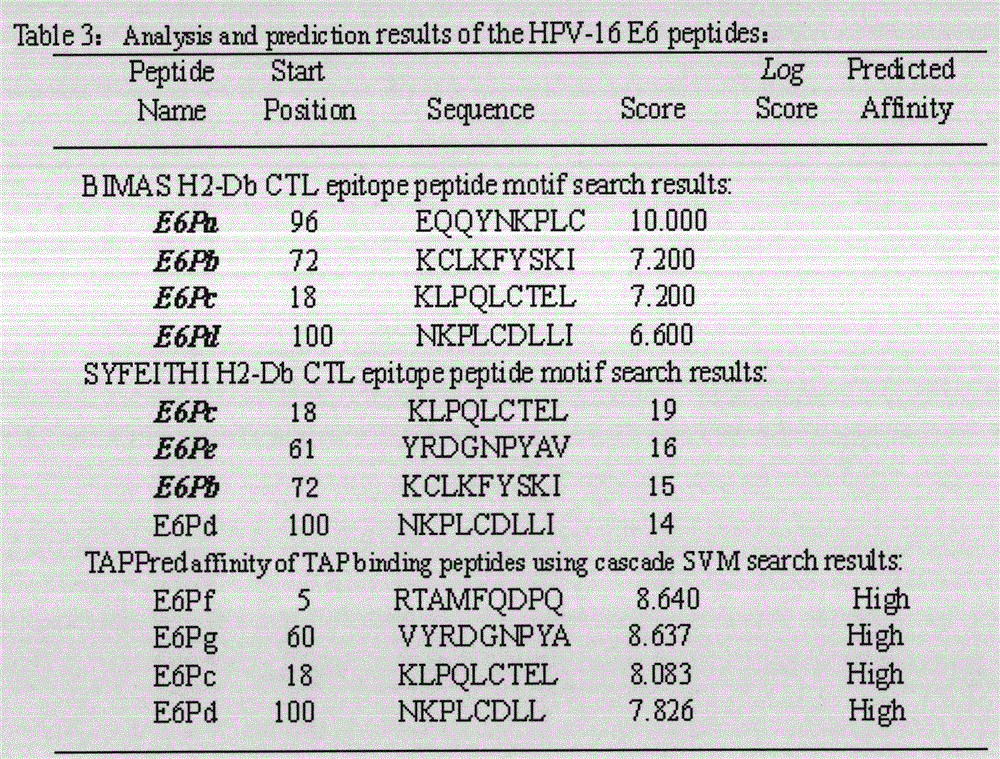
Screening and verifying of human papilloma virus (HPV) 16 tripeptide vaccine and construction of tumor animal model for continually expressing HPV16 E5, E6 and E7
ActiveCN102343103ASignificant therapeutic or preventive effectSuppression of tumorigenesisViral antigen ingredientsGenetic material ingredientsAntigen Processing CellAdeno associate virus
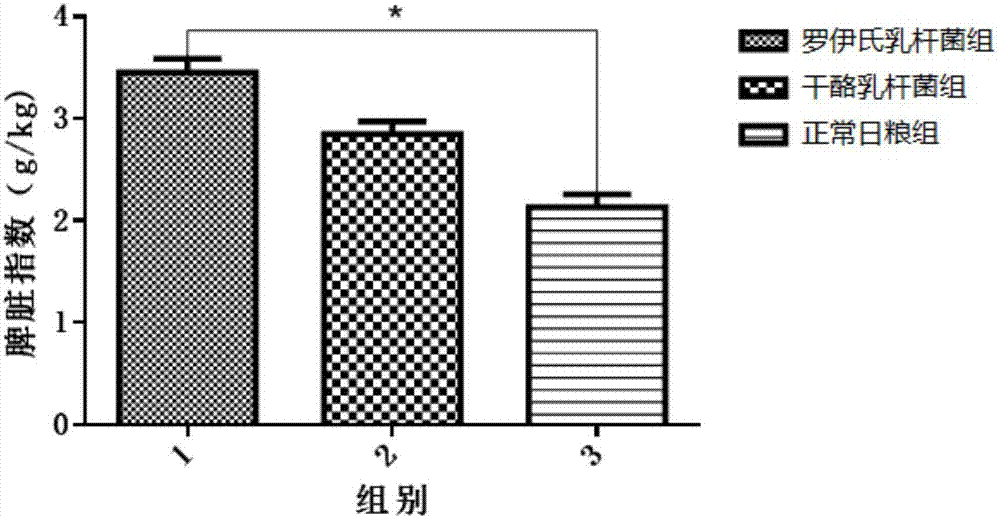
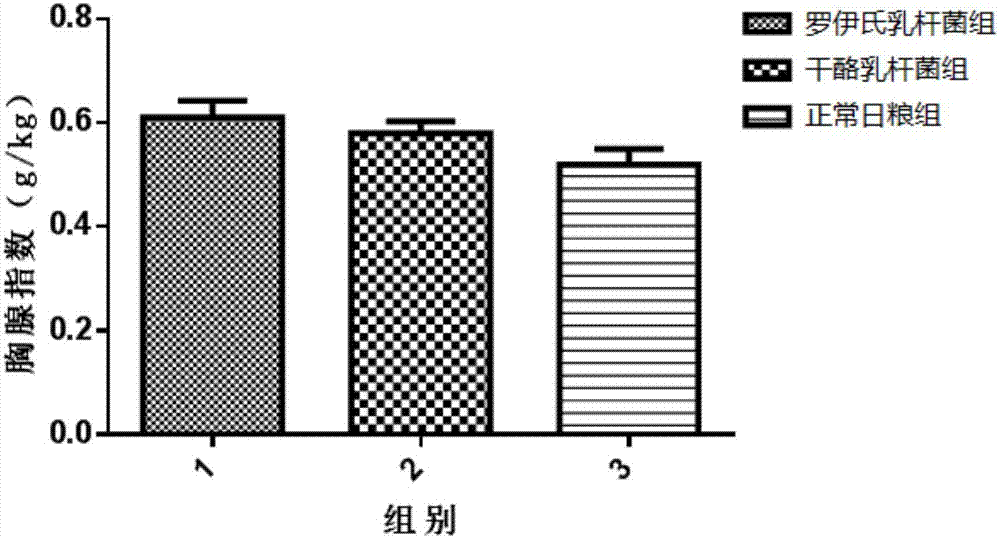
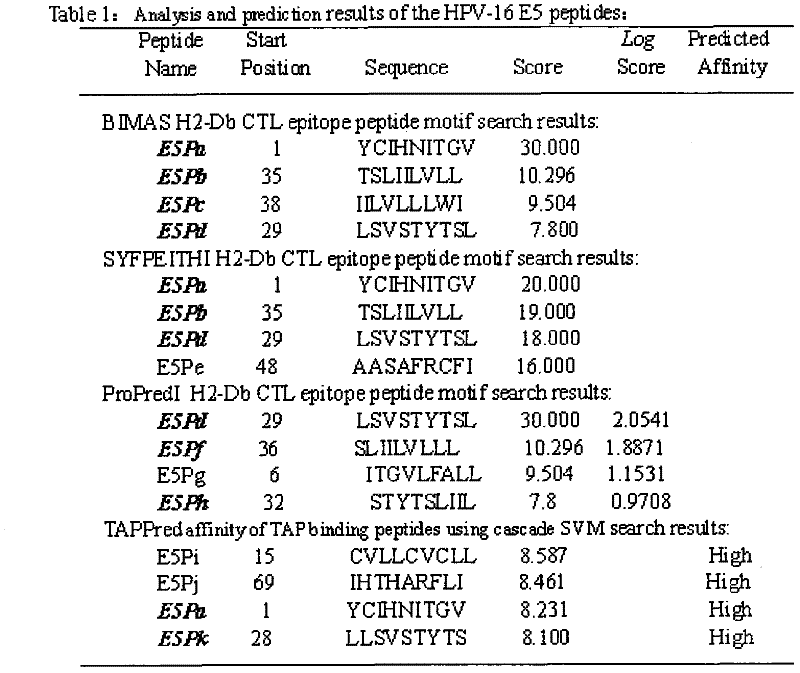
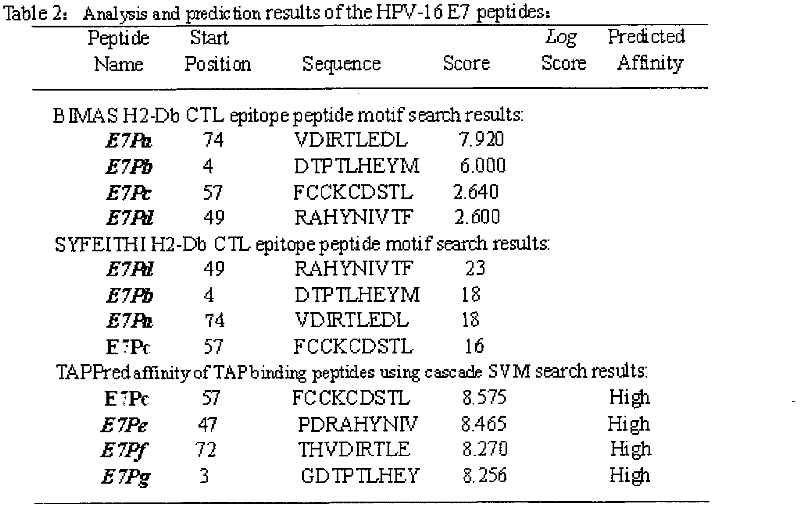
The invention discloses screening and verifying of a human papilloma virus (HPV) 16 tripeptide vaccine and a construction scheme of a tumor animal model for continually expressing HPV16 E5, E6 and E7. Polypeptide fragments with high scores are screened with a method for analyzing polypeptide fragments specific to a transporter associated with antigen processing (TAP) in combination with bioinformatics, a tripeptide vaccine core consists of three fragments with strongest immune effects obtained by performing in-vivo and in-vitro tests, and an HPV16 vaccine with preventing and treating functions is constructed in combination with a CPG adjuvant. Simultaneously, a tumor animal model for continually expressing HPV16 E5, E6 and E7 is constructed. The invention is technically characterized in that: by stably integrating HPV16E5 into a TC-1 cell through an adeno-associated virus expression system and injecting the modified TC-1 cell into a C57BL / 6 mouse body, a tumor can be formed successfully.
Owner:马丁
Herpes virus vectors for dendritic cells
InactiveUS20050249707A1Highly efficient gene deliveryEfficient implementationBiocideGenetic material ingredientsInfected cellDisease
An attenuated herpes virus capable of efficiently infecting a dendritic cell without preventing antigen processing occurring within the infected cell. The attenuated herpes virus and dendritic cells infected with the virus are useful immunotherapeutic methods of treating disease.
Owner:BIOVEX LTD
Modular antigen transporter molecules (MAT molecules) for modulating immune reactions, associated constructs, methods and uses
By adding the novel developed modular antigen transporter molecules (MAT molecules) and other instruments related thereto, the immune system of an individual can be effectively modulated in a targetedmanner. The MAT molecules contain a translocation module which transports the MAT molecule inside the cell, an active intracellular targeting molecule resulting in intracellular transport of the MATmolecule to the organelles of the cell which are responsible for antigen processing and an antigen module which determines the specificity of the immune response modulated by the MAT molecule. Optionally, other modules such as tag modules or spacer modules can be contained in the MAT molecule. The invention comprises the inventive MAT molecules, antibodies which are produced using said MAT molecules and therapeutic agents and diagnostic reagents which are produced with MAT molecules. The invention also comprises applications containing said MAT molecules, antibodies, therapeutic agents and diagnostic reagents.
Owner:BOEHRINGER LNGELHEIM VETMEDICA GMBH
Medicine as well as preparation method and application thereof
ActiveCN107050463AEnhanced antigen presentationImprove the effect of tumor treatmentPeptide/protein ingredientsCarrier-bound antigen/hapten ingredientsAntigen processingAutophagic death
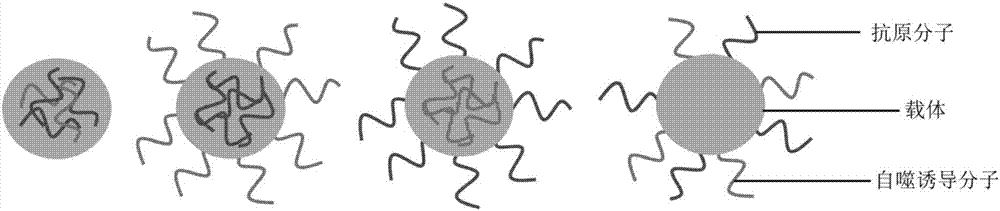
The invention relates to a medicine as well as a preparation method and application thereof and in particular relates to a medicine with an antigen processing improving function as well as a preparation method and application thereof. The medicine is prepared from a cell autophagy induction molecule, a tumor antigen molecule and a carrier, wherein the autophagy induction molecule and the tumor antigen molecule are connected onto the carrier. According to the medicine, the cell autophagy induction molecule and the tumor antigen molecule are connected onto the same carrier and have the synergistic interaction effect; compared with the effect of a manner of independently connecting the molecules with the carrier and entering cells respectively, the effect of the two molecules is remarkably enhanced; autophagy is effectively induced so that antigen presentation is enhanced; finally, the aim of improving the effect of treating tumors is realized; moreover, autophagy is enhanced by the medicine so that the antigen presentation is improved and the medicine provides a new thought for development of tumor immunotherapy.
Owner:THE NAT CENT FOR NANOSCI & TECH NCNST OF CHINA
Lipids as synthetic vectors to enhance antigen processing and presentation ex-vivo in dendritic cell therapy
ActiveUS20190358319A1Increased proliferationImprove viabilityViral antigen ingredientsMammal material medical ingredientsTumor targetRegulatory T cell
Owner:PDS BIOTECH
Peptides
The present invention provides a peptide at least partially derivable from human Thyroid Stimulating Hormone Receptor (TSHR) which peptide is capable of binding to an MHC molecule in vitro and being presented to a T cell without further antigen processing. The present invention also relates to the use of such peptides for the prevention or suppression of activating autoantibody formation in Graves' Disease.
Owner:WORG PHARM (ZHEJIANG) CO LTD
Peptides
ActiveUS20180327463A1Peptide/protein ingredientsVertebrate antigen ingredientsDiseaseMolecular binding
The present invention provides a peptide at least partially derivable from human Thyroid Stimulating Hormone Receptor (TSHR) which peptide is capable of binding to an MHC molecule in vitro and being presented to a T cell without further antigen processing. The present invention also relates to the use of such peptides for the prevention or suppression of activating autoantibody formation in Graves' Disease.
Owner:WORG PHARM (ZHEJIANG) CO LTD
Dendritic cell targeted affinity peptide TY peptide and application thereof
ActiveCN109021078APromote maturityImprove intake efficiencyPowder deliveryPeptidesDendritic cellTumor therapy
Belonging to the tumor therapy protein related technical field, the invention specifically relates to a dendritic cell (DC) targeted affinity peptide TY peptide and application thereof. The TY peptideis 12 peptide, and the specific amino acid sequence is TITHFQFGPTVY. Based on the TY peptide, the invention further designs a novel nano-transportation system capable of targeting DC on the basis ofMSN, and loads the model antigen OVA and immunoadjuvant CpG to prepare the nano-carrier MSN(mesoporous silica nanoparticles)-TY / OVA / CpG successfully. Experiments indicate that: the MSN-TY / OVA / CpG canbe effectively ingested by immature DC, thus improving the efficiency of antigen ingestion by DC, promoting the maturation of DC and effectively activating DC, also effectively promoting antigen processing and presentation, stimulating secretion of cytokines, and further triggering antigen-specific CTL immunoreaction and anti-tumor immunoreaction.
Owner:ZHENGZHOU UNIV
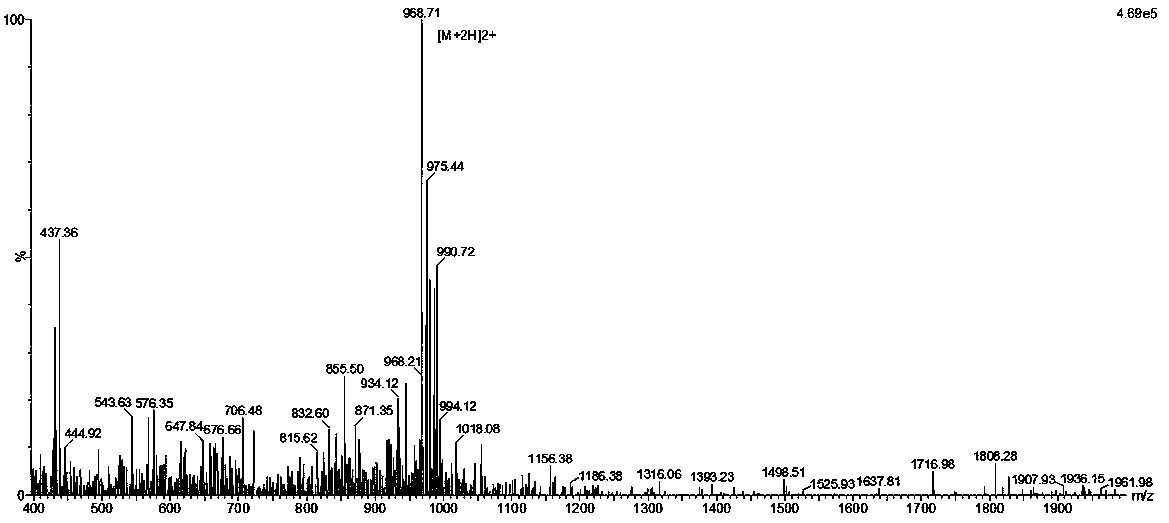
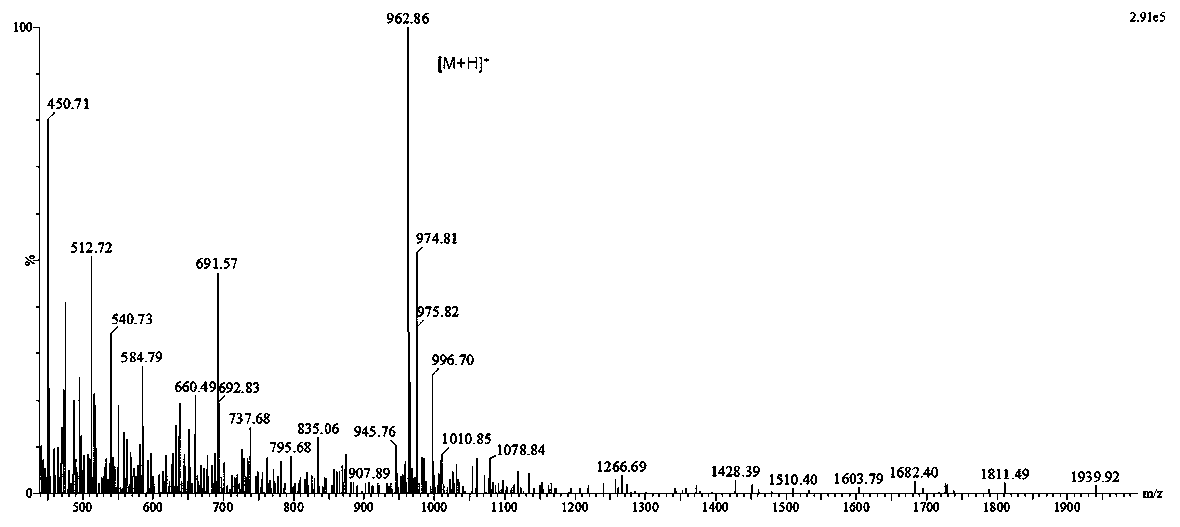
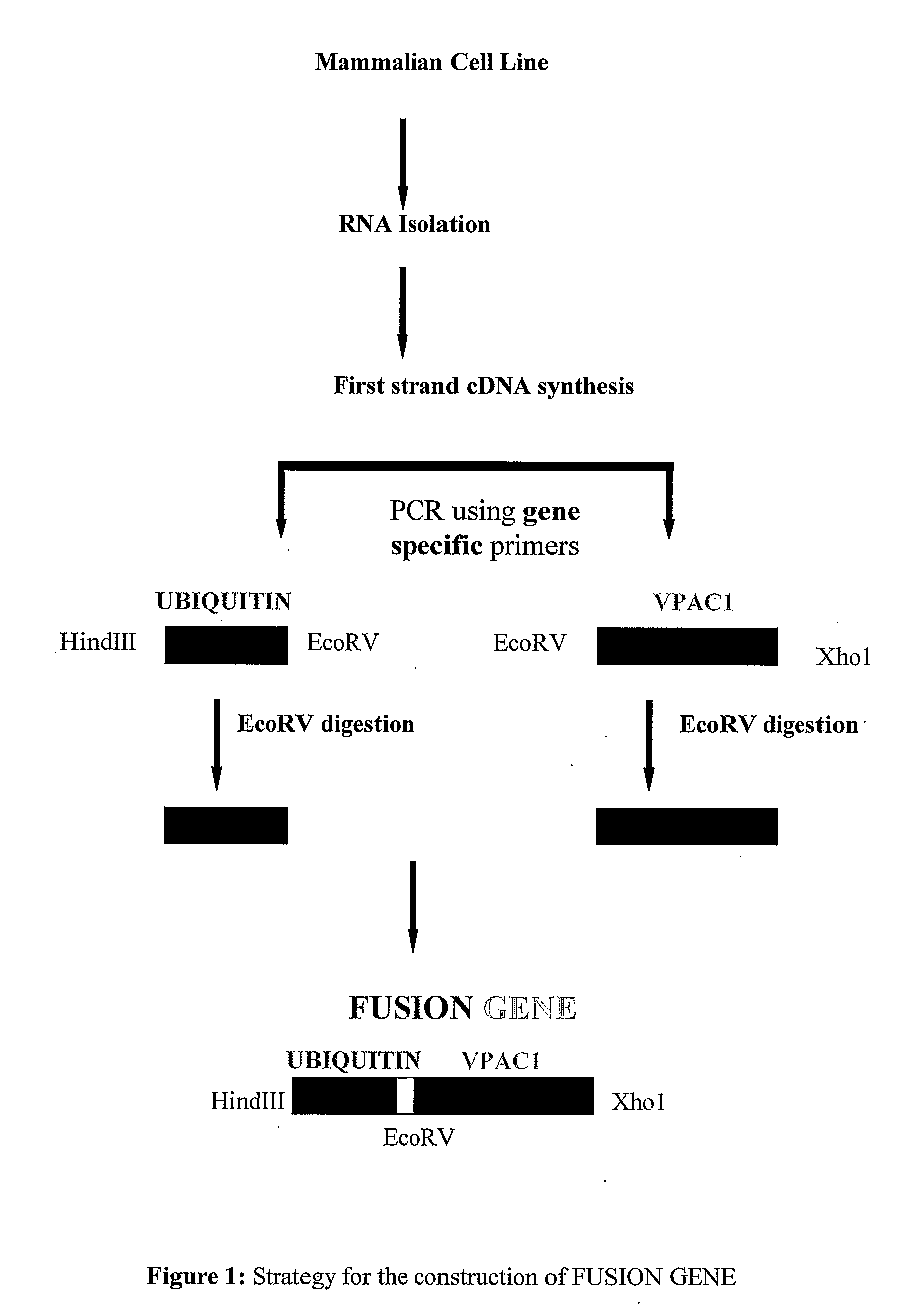
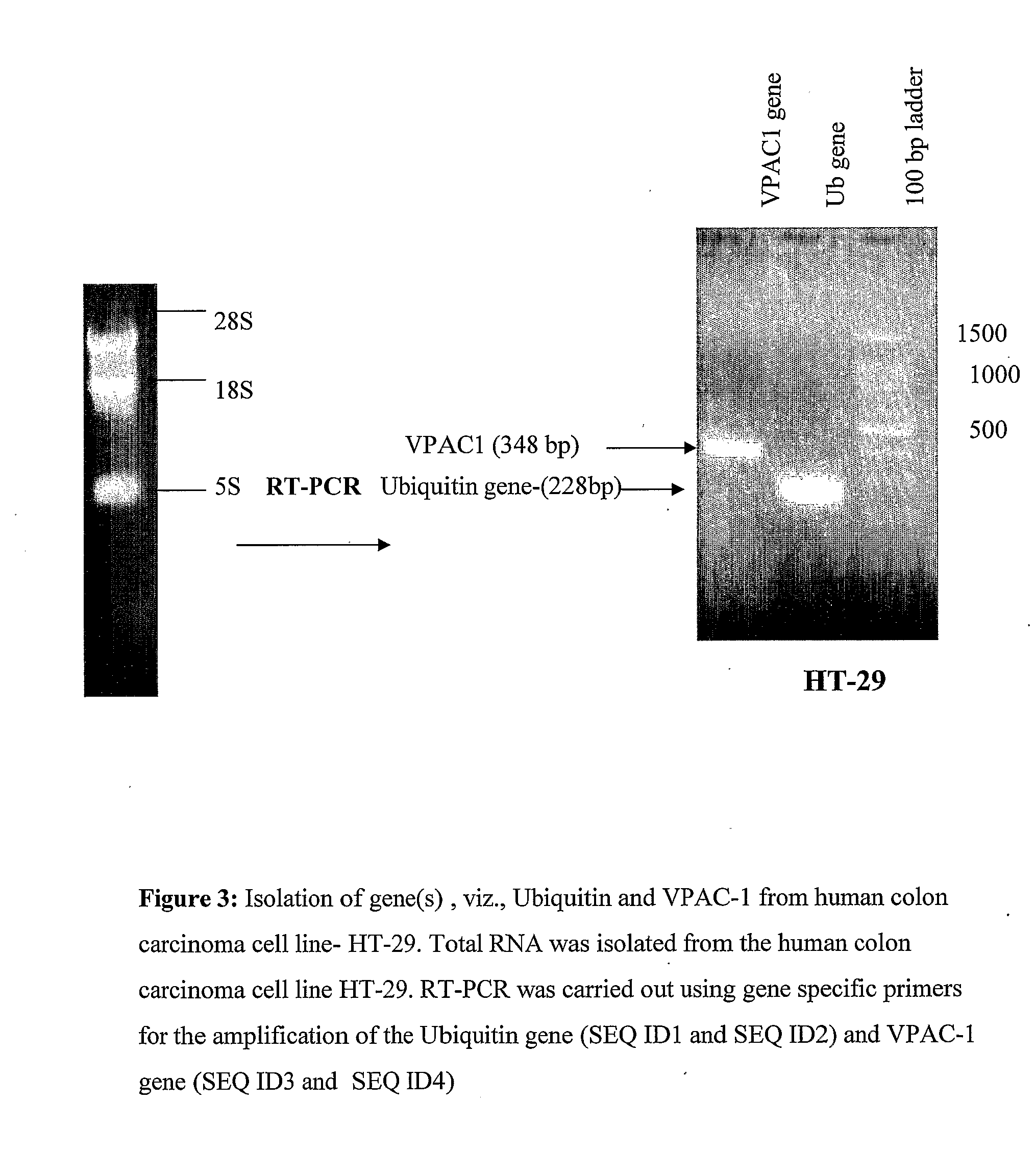
DNA Vaccine for Cancer Therapy
InactiveUS20090221682A1Marked suppression of tumor growthImprove clinical efficacyPolypeptide with localisation/targeting motifOrganic active ingredientsUbiquitin ligaseVasoactive intestinal peptide
The invention relates to a fusion gene useful as a therapeutic and prophylactic vaccine against cancer. The fusion gene contains a DNA encoding ubiquitin gene fused to a second DNA encoding growth factor receptors or fragment thereof, over expressed in various types of cancer. In particular, the invention is illustrated by a recombinant fusion gene comprised of mutated ubiquitin gene and modified extra cellular domain of vasoactive intestinal peptide / pituitary adenylate cyclase activating polypeptide receptor-1 (VPAC1). Immunization with the vaccine will break the self-tolerance for the tumor antigen through effective antigen processing and presentation, leading to retardation / regression of tumor growth.
Owner:DABUR PHARM LTD
Single-domain antibody capable of recognizing HLA-A2/RMFPNAPYL
ActiveCN107698681AUnique identification abilityHybrid immunoglobulinsImmunoglobulins against animals/humansAntigenNucleotide
The invention discloses a single-domain antibody capable of recognizing HLA-A2 / RMFPNAPYL and CDR1, CDR2, and CDR3 sequences having a specific recognition effect. A fusion protein is characterized by being prepared by abovementioned amino acid sequences and at least one polypeptide having a recognition function. A multi-specific or multifunctional molecule comprises the single-domain antibody. A nucleic acid molecule encodes the polypeptide molecule. A carrier comprises the nucleic acid sequence. The nucleotide sequence or at least part of sequence of a protein expressed by the nucleic acid molecule can be expressed by a proper expression system so as to obtain a corresponding protein or polypeptide. A host cell comprises a nucleic acid sequence that expresses the abovementioned protein. The single-domain antibody can recognize an artificially synthesized HLA-A2 / RMFPNAPYL compound, can be combined with a HLA-A2 / RMFPNAPYL compound that is expressed on the surface of tumor cells and is prepared from natural antigen, and can be used to develop a product for treating related tumors.
Owner:SHENZHEN BEIKE BIOTECH
ERAAP modulators regulate immune responses
InactiveUS7005269B2Organic active ingredientsAnimal cellsComplementarity determining regionAntigen processing
An immune response is modulated by selectively inhibiting ERAAP (an acronym for ER aminopeptidase associated with antigen processing) and confirming a resultant immune response modulation. More particularly, the method comprises contacting a patient determined to be in need of immune response modulation with a physiologically acceptable dosage composition comprising an effective amount of an inhibitor of ERAAP activity; confirming a resultant inhibition of said ERAAP activity and confirming a resultant immune response modulation in the patient. A variety of selective inhibitors are shown to be effective, including amino thiols, such as leucine thiol, ERAAP-specific antibody complementarity-determining region, and an ERAAP-specific siRNA.
Owner:RGT UNIV OF CALIFORNIA
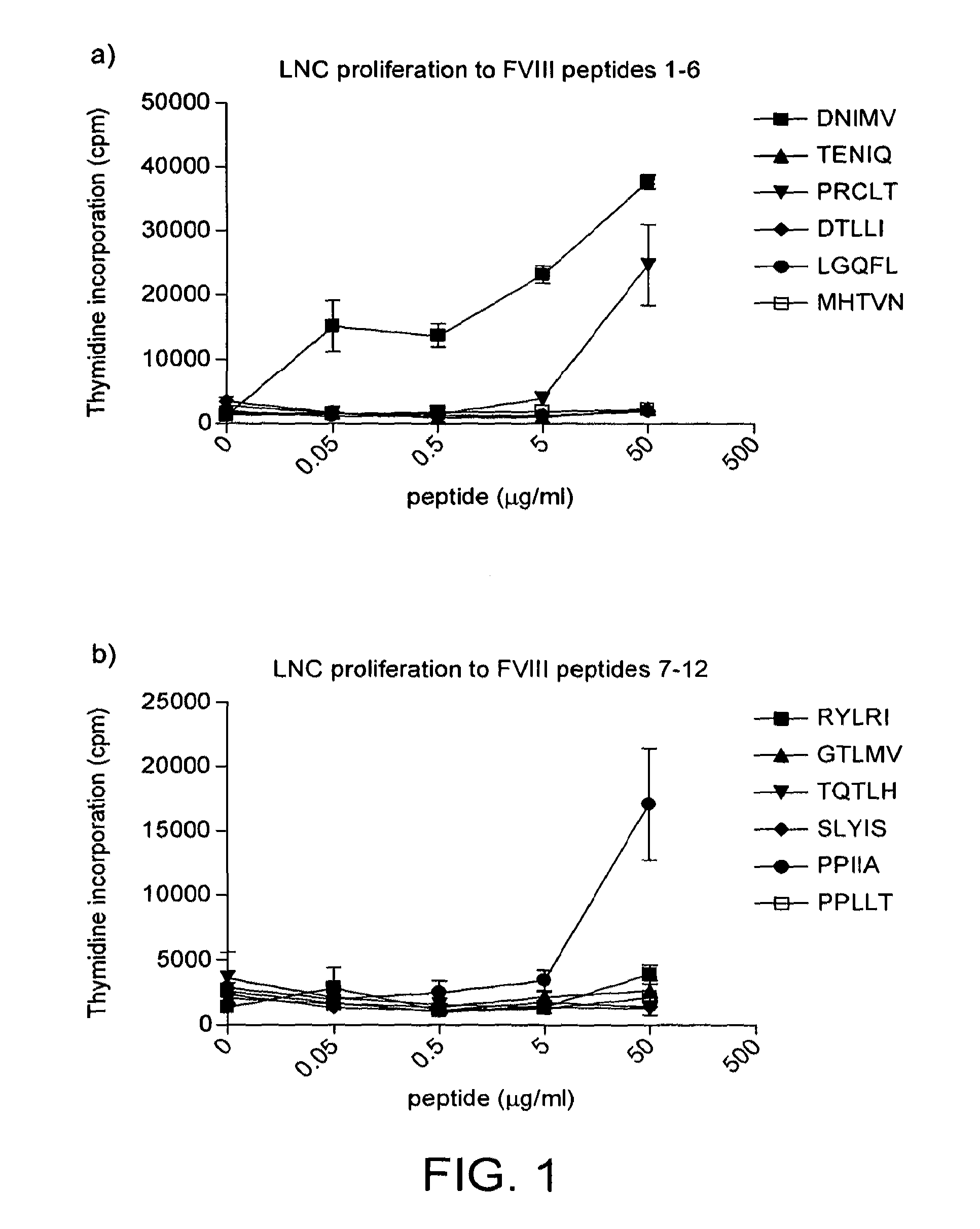
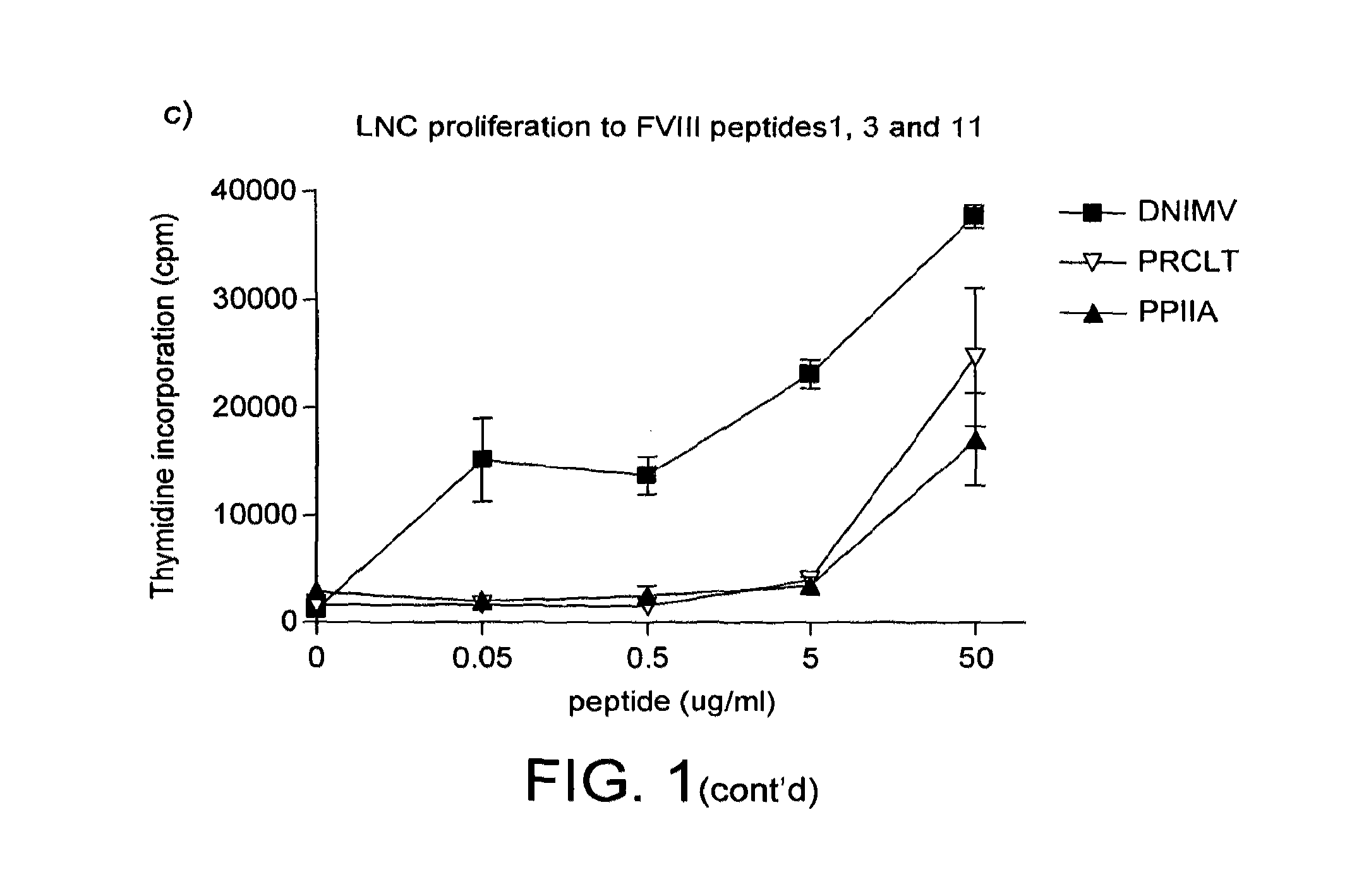
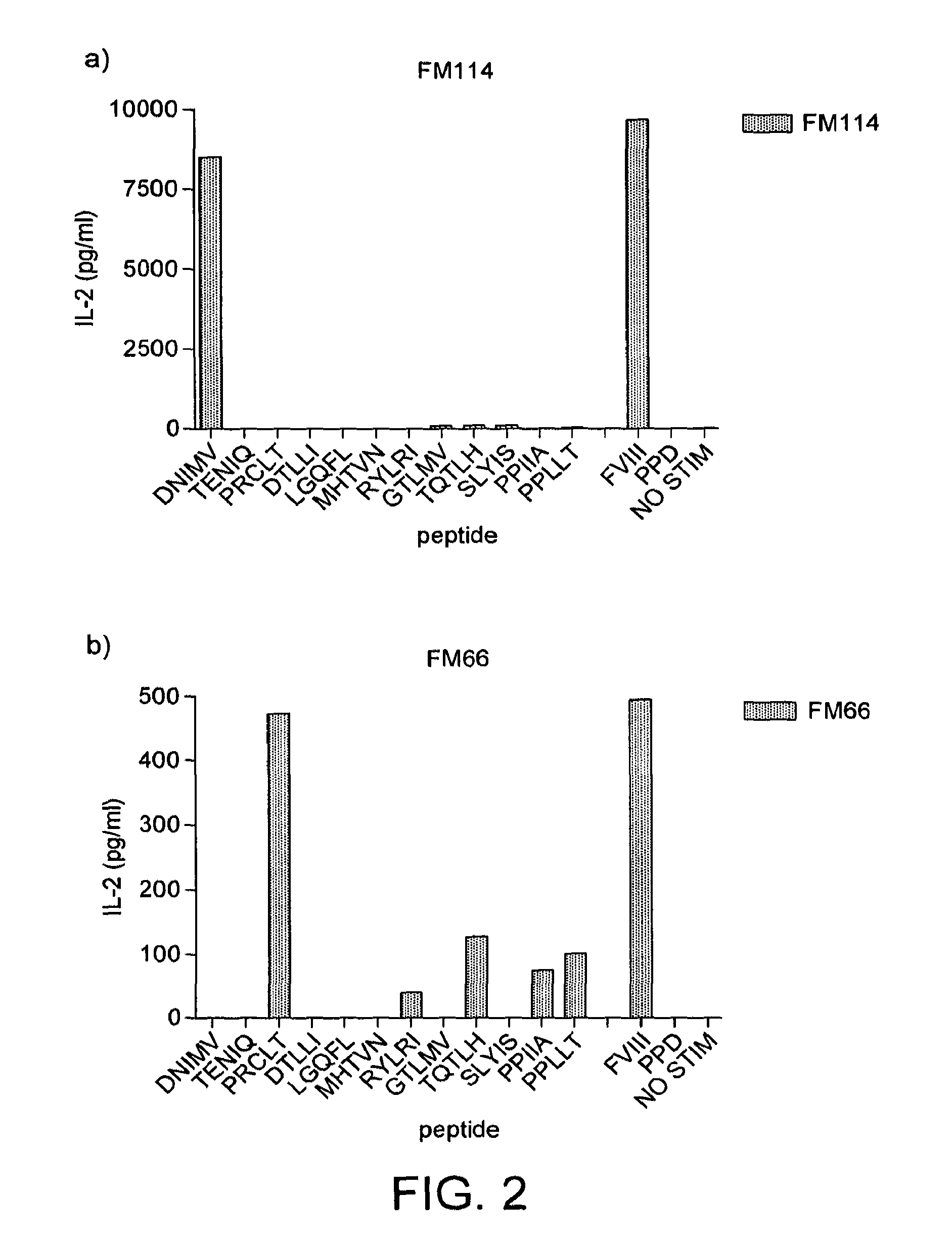
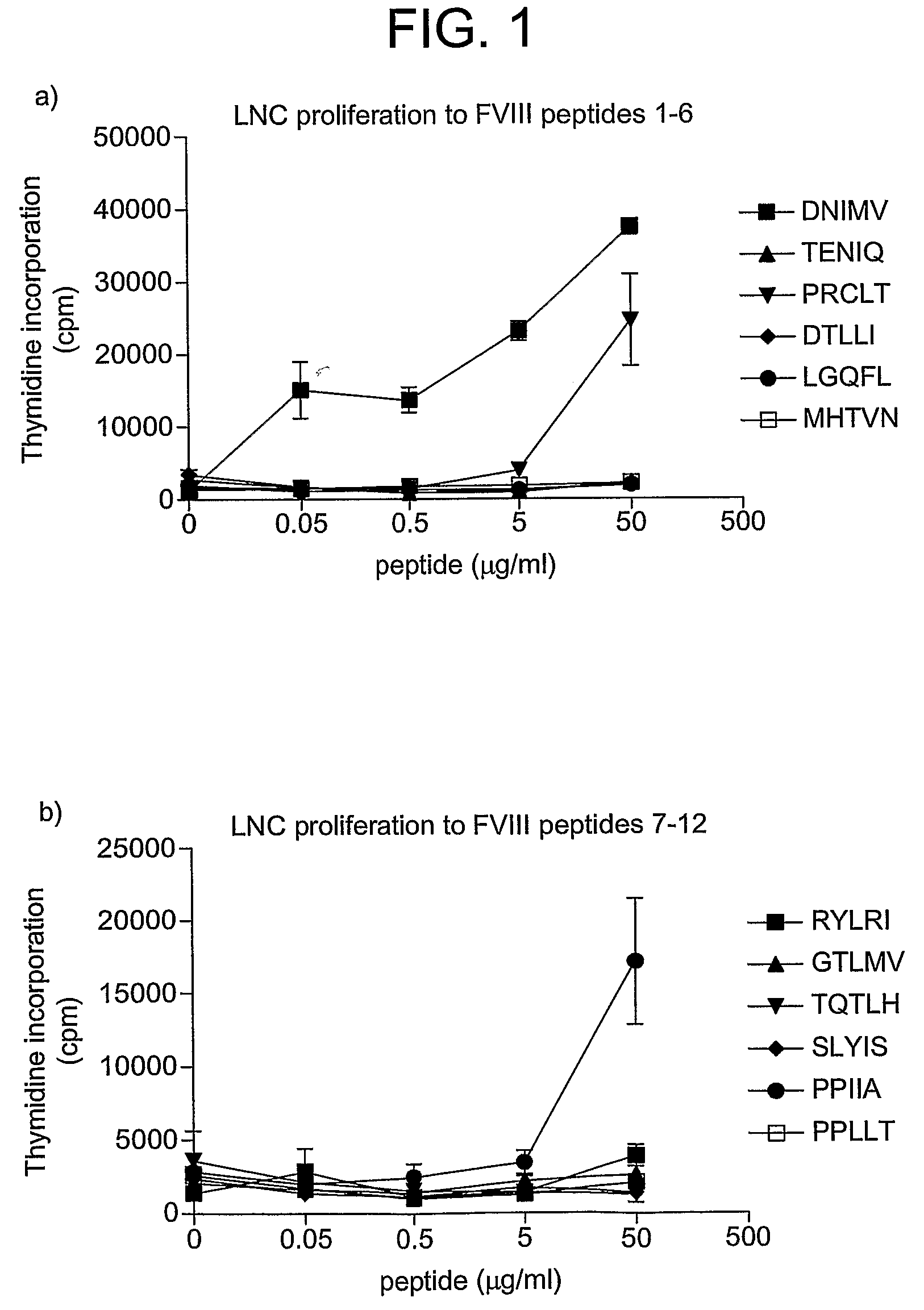
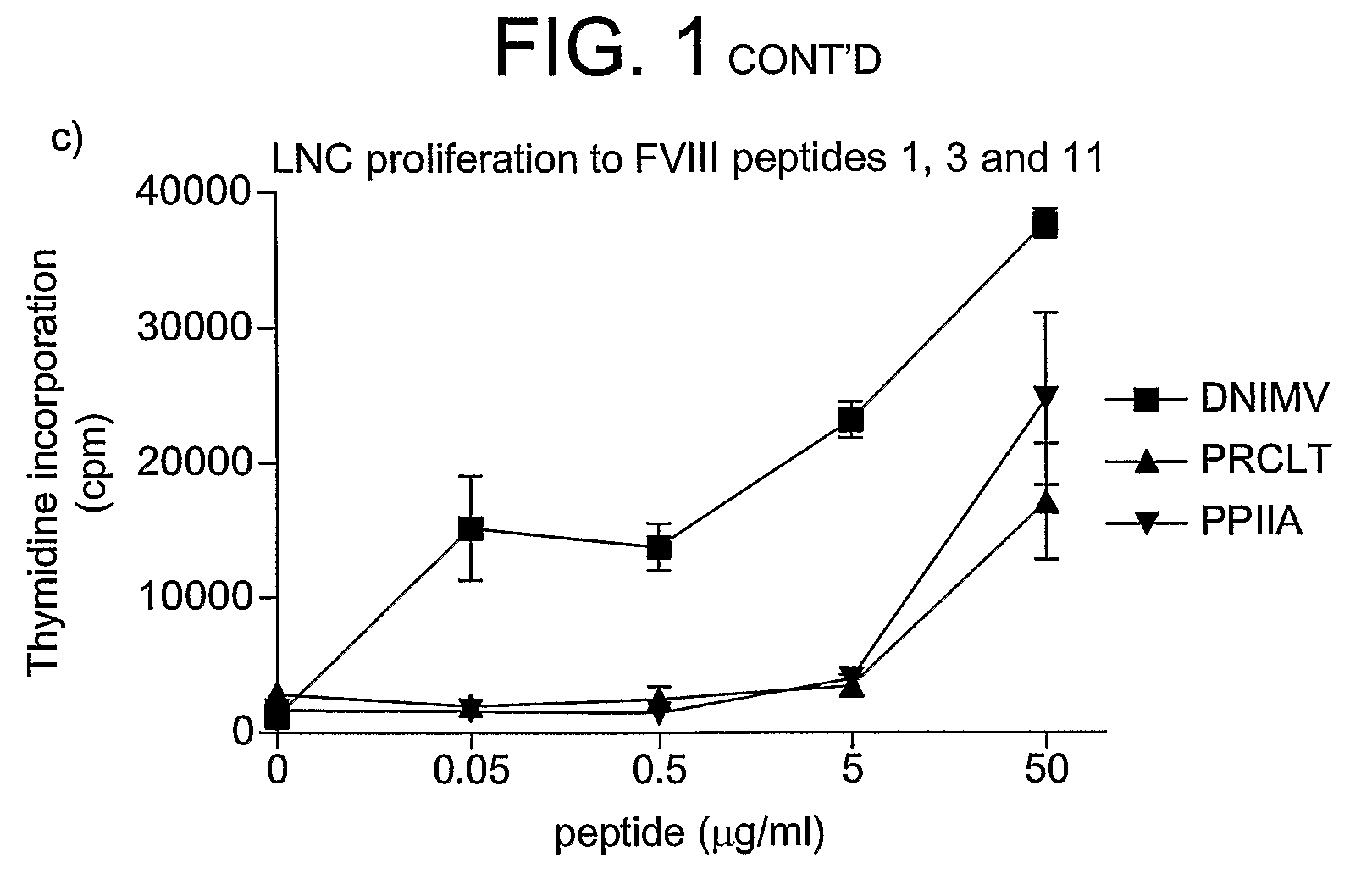
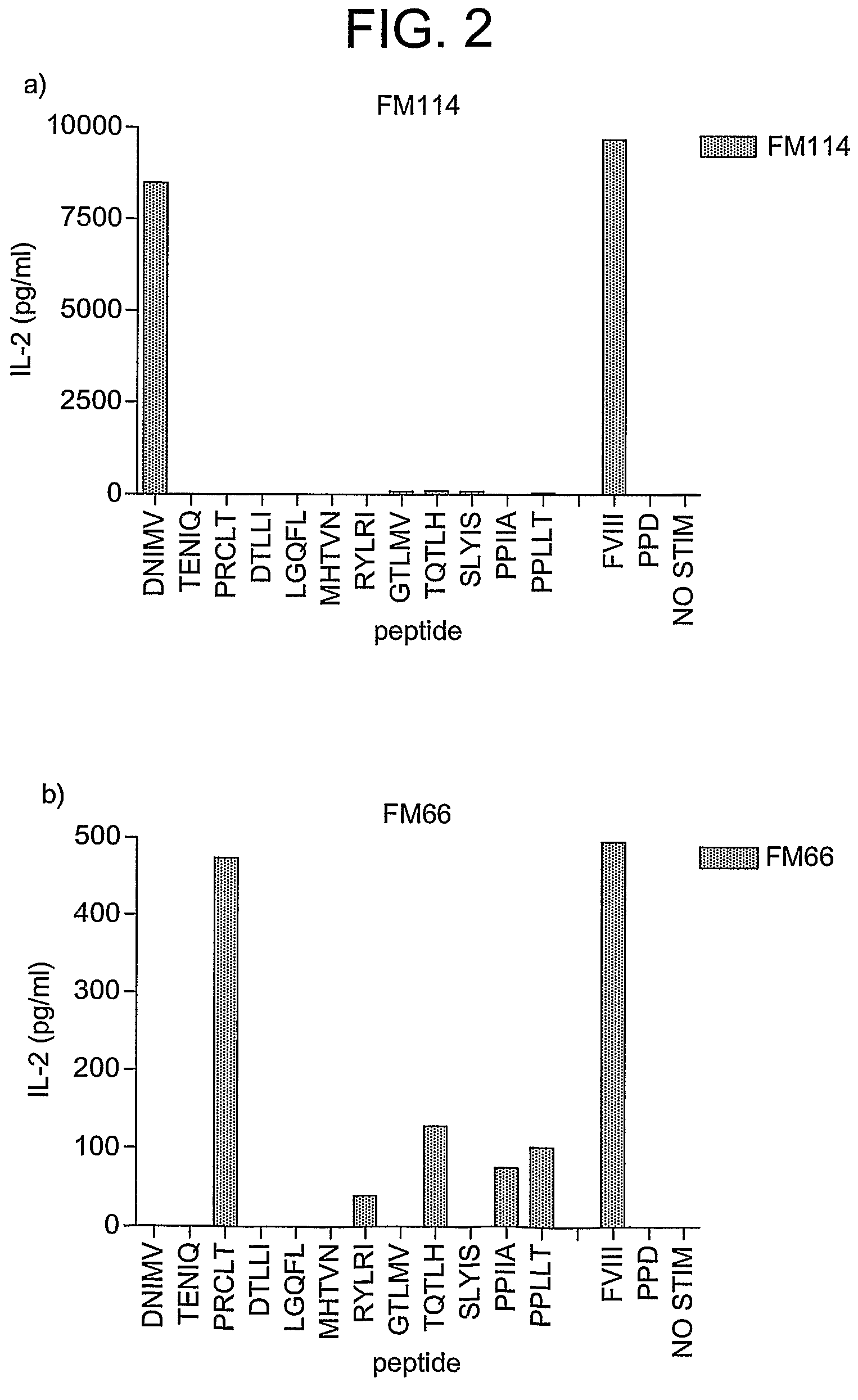
Modified factor VIII peptides
InactiveUS8703705B2Suppressing and preventing and reducing developmentFactor VIIPeptide/protein ingredientsAntigen processingT cell
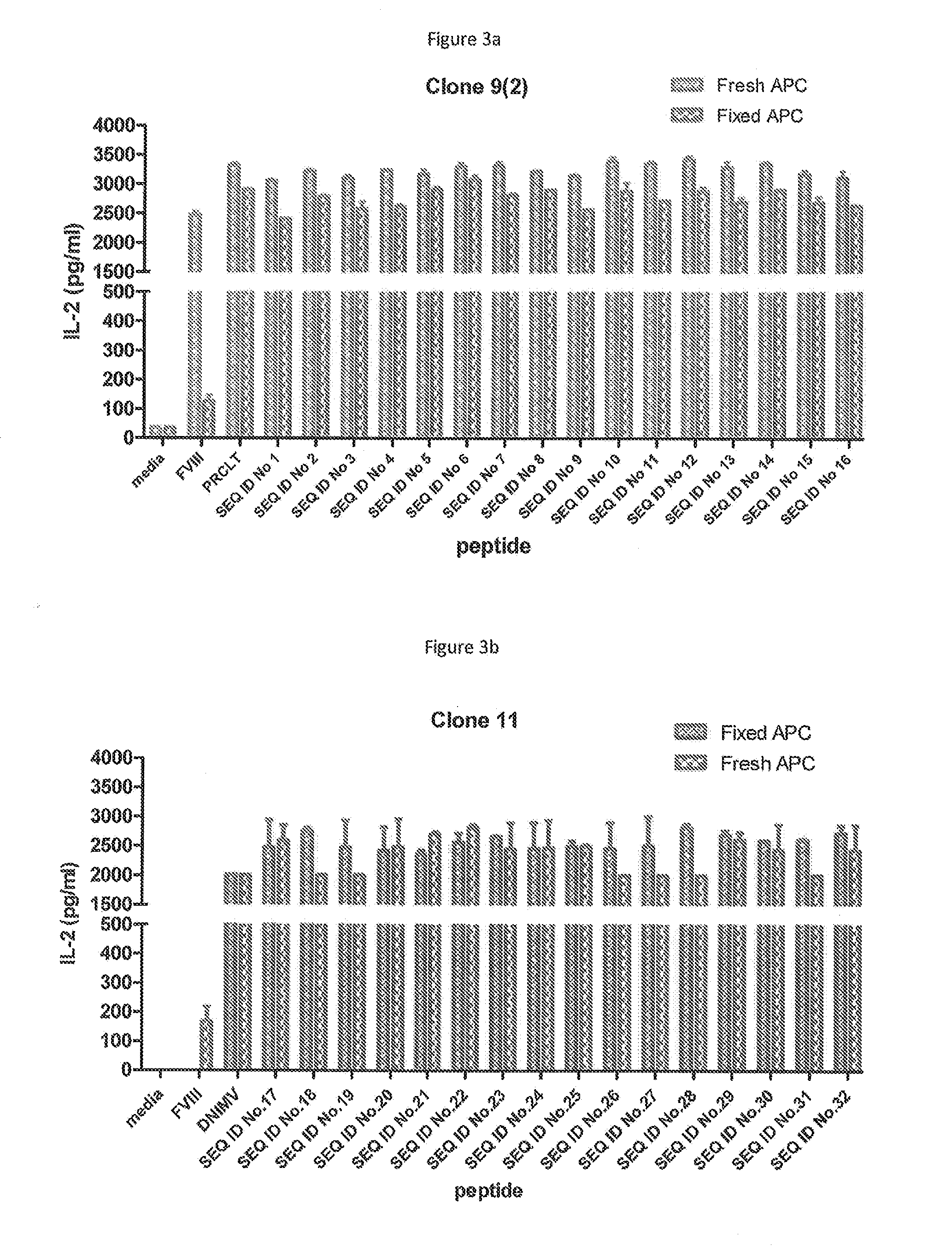
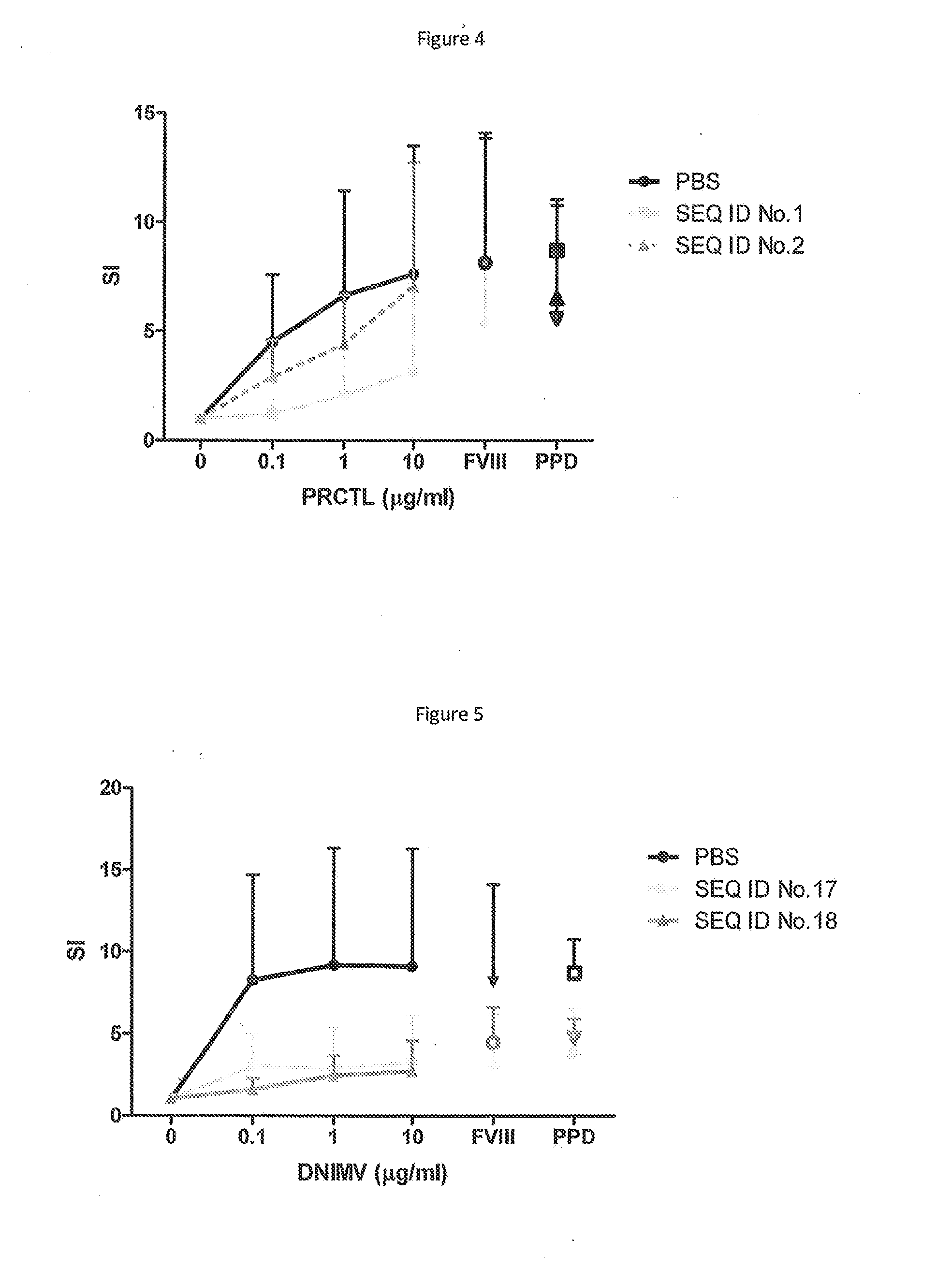
The present invention provides peptides at least partly derivable from FVIII which are capable of binding to an MHC class II molecule without further antigen processing and being recognized by a factor VIII specific T cell. In particular, the present invention provides a peptide comprising or consisting of the sequence EDNIMVTFRNQASR. The present invention also relates to the use of such a peptide for the prevention or suppression of inhibitor antibody formation in haemophilia A and / or acquired haemophilia.
Owner:APITOPE INT
FVIII peptides and their use in tolerising haemophiliacs
ActiveUS8445448B2Suppressing and preventing and reducing developmentFactor VIIPeptide/protein ingredientsHemophilia patientAcquired haemophilia
The present invention provides a peptide comprising a core residue sequence derivable from human FVIII which peptide is capable of binding to an MHC class II molecule without further antigen processing. The present invention also relates to the use of such peptides for the prevention or suppression of inhibitor antibody formation in haemophilia A and / or acquired haemophilia.
Owner:WORG PHARM (ZHEJIANG) CO LTD
Mycobacterium mutants for vaccines with improved protective efficacy
InactiveUS8685415B2Improved vaccine efficiencyGood curative effectAntibacterial agentsBiocideAbnormal macrophageMannosyltransferase
Tuberculosis (TB) is a major health problem and currently, the only licensed TB vaccine is Mycobacterium bovis Bacille Calmette-Guerin (M. bovis BCG). In the present invention, mutation of mycobacterial components reportedly involved in phagosome maturation inhibition was evaluated for vaccine purposes, as such mutations should result in better vaccine antigen processing and presentation. Thus, BCG mutants in genes coding for ManLAM capping α-1,2-mannosyltransferases and the PI3P phosphatase SapM were evaluated as TB vaccines in a stringent mouse model. Vaccination with both ManLAM capping mutants and the SapM mutant resulted in significantly longer survival as compared to non-vaccinated mice, whereas vaccination with the parental BCG did not. Moreover, mice vaccinated with the SapM mutant survived significantly longer than mice vaccinated with the parental BCG. The mutant BCG strains showed unaltered phagocytosis, replication, lysosome colocalization and oxidant activity in macrophages and similarly induced autophagy in the latter. Additionally, replication and granuloma formation in mice was unaffected, indicating BCG-equivalent safety of these vaccines.
Owner:VLAAMS INTERUNIVERSITAIR INST VOOR BIOTECHNOLOGIE VZW +2
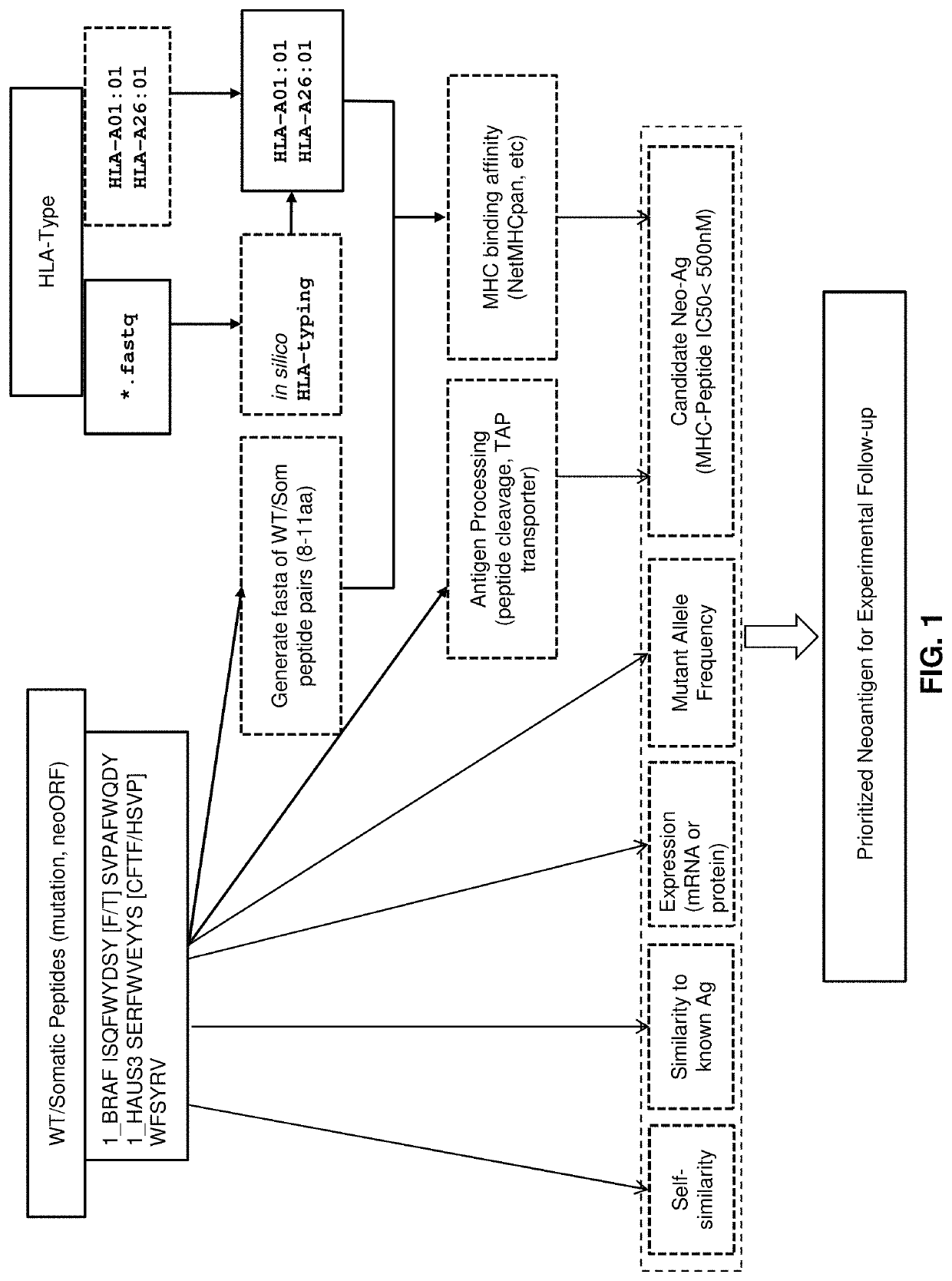
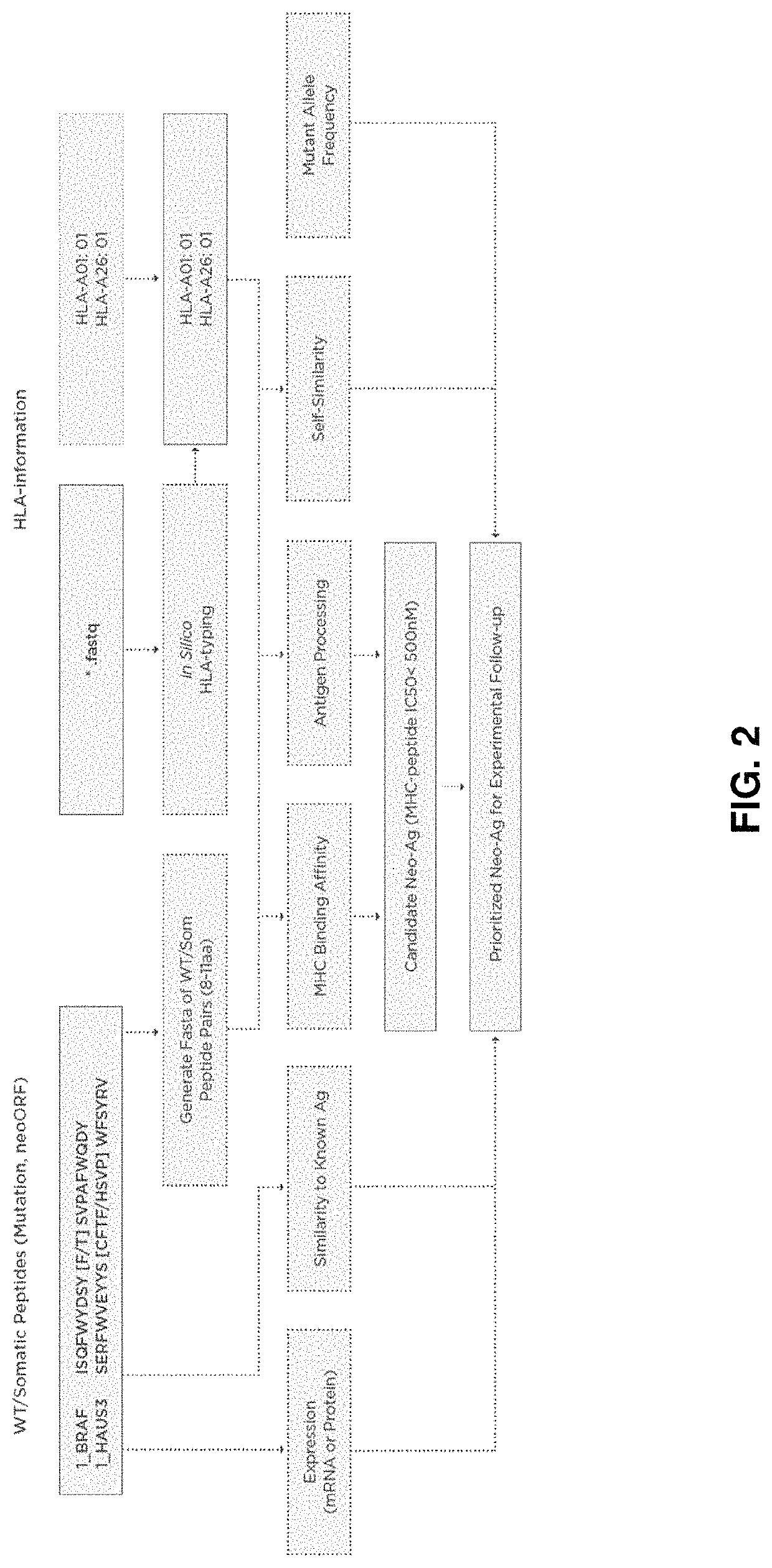
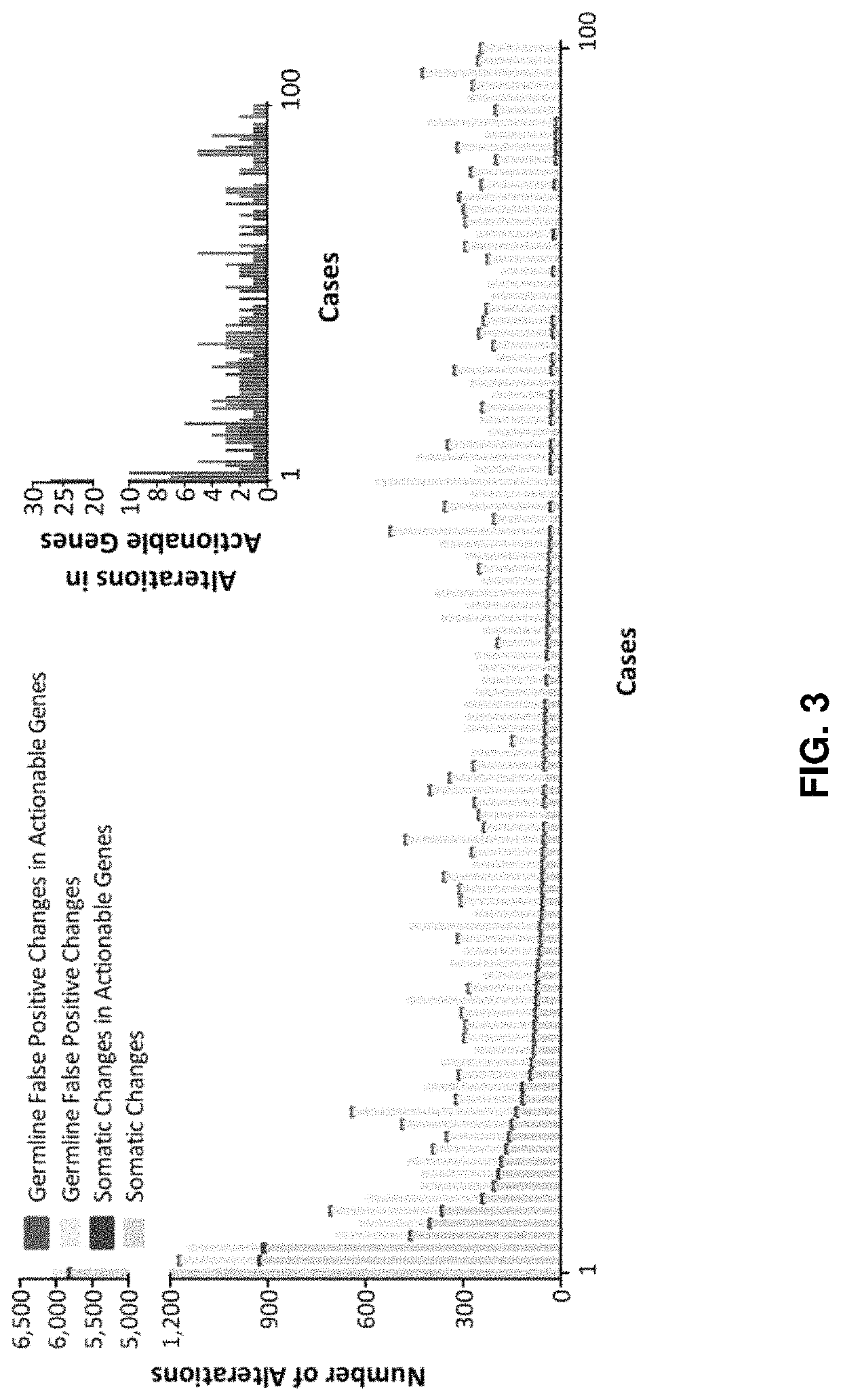
Neoantigen treatment prioritization using multivariate analysis based on: HLA genotype, self-similarity, similarity to known antigens, antigen expression levels and mutant allele frequency
ActiveUS10563266B2Shorten the timeSave moneyMolecular entity identificationMolecular designMutant alleleCancer immunology
Owner:PERSONAL GENOME DIAGNOSTICS INC
Myelin oligodendrocyte glycoprotein peptides
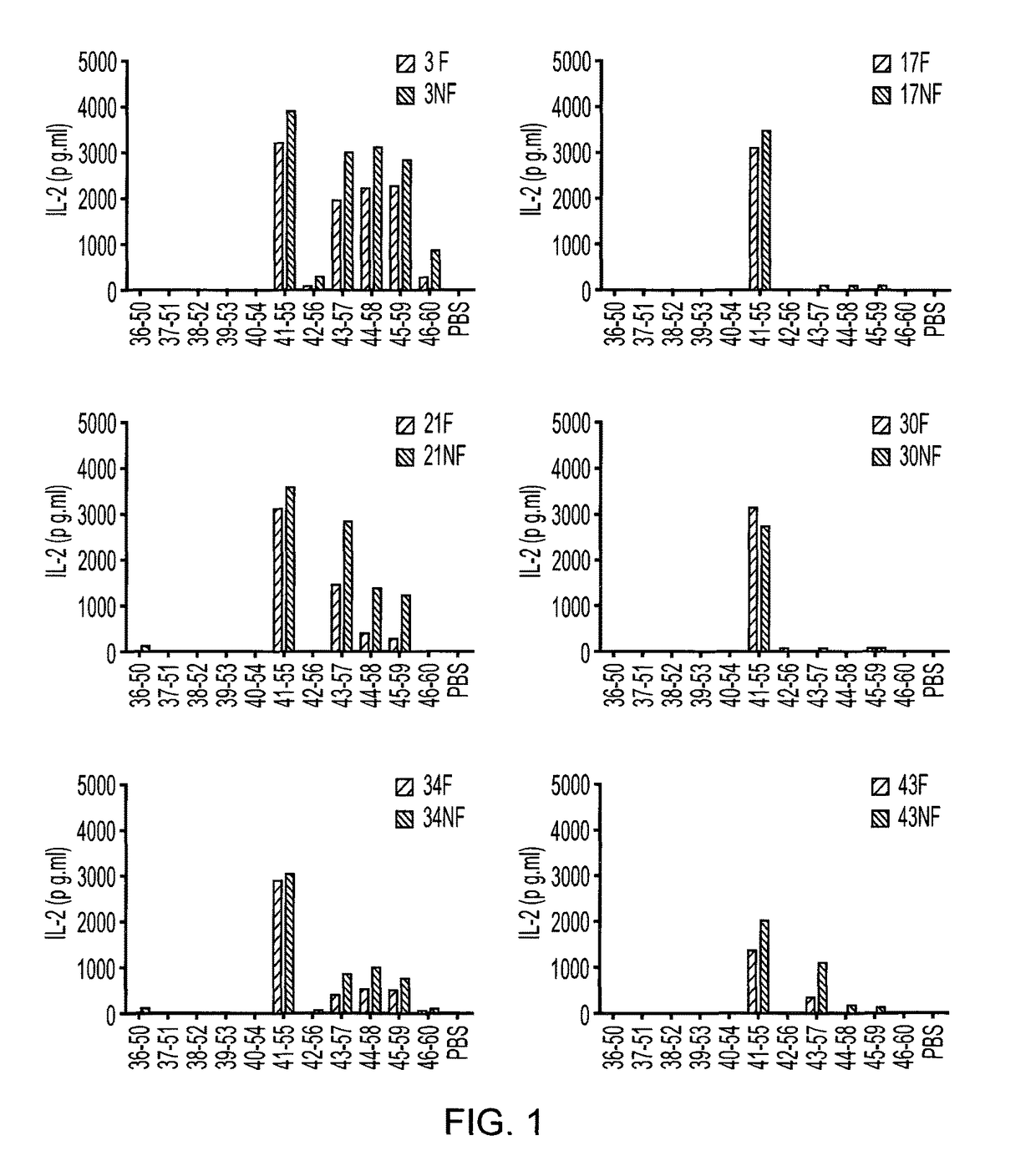
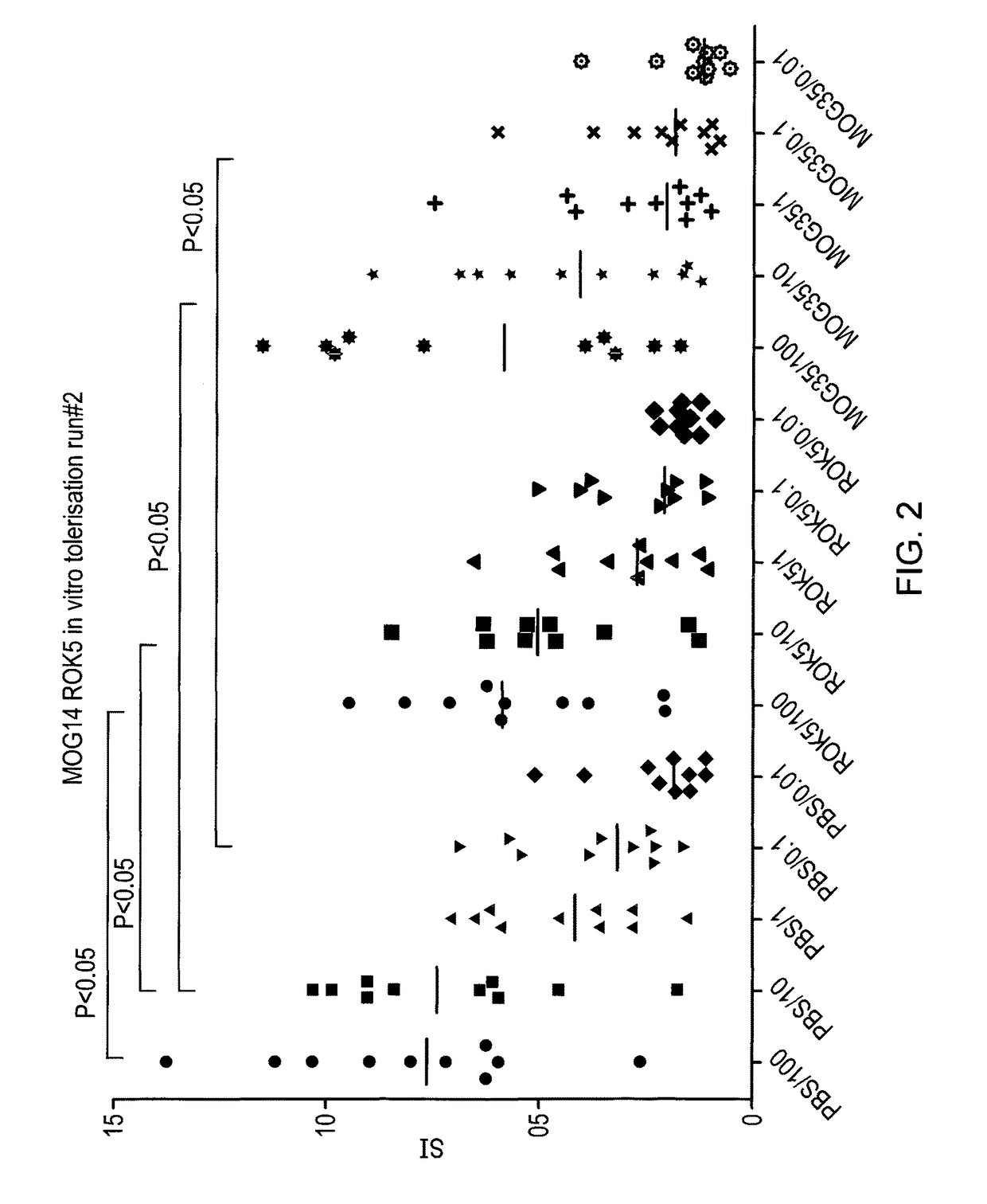
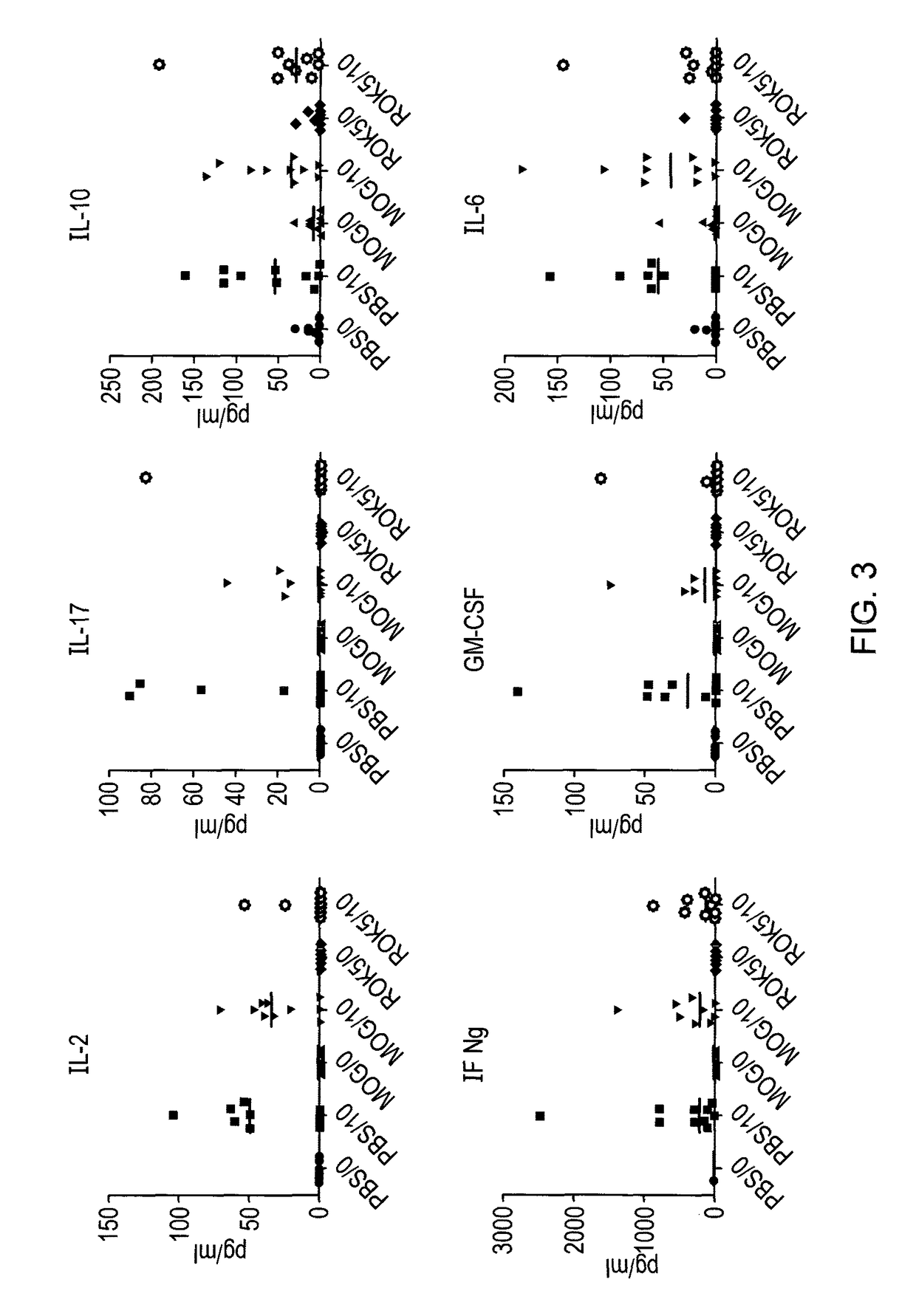
There is provided a peptide which is capable of binding to an MHC molecule in vitro and being presented to a T cell without antigen processing (i.e. an apitope) which peptide comprises a portion of the region 40-60 of myelin oligodendrocyte glycoprotein (MOG). In particular there is provided an apitope which is selected from the following myelin oligodendrocyte glycoprotein peptides: MOG 41-55, 43-57, 44-58 and 45-59. There is also provided the use of such a peptide in a pharmaceutical composition and a method to treat and / or prevent a disease using such a peptide.
Owner:WORG PHARM (ZHEJIANG) CO LTD
Modified fviii apitope peptides
InactiveUS20150306192A1Suppressing and preventing and reducing developmentFactor VIIPeptide/protein ingredientsAntigen processingT cell
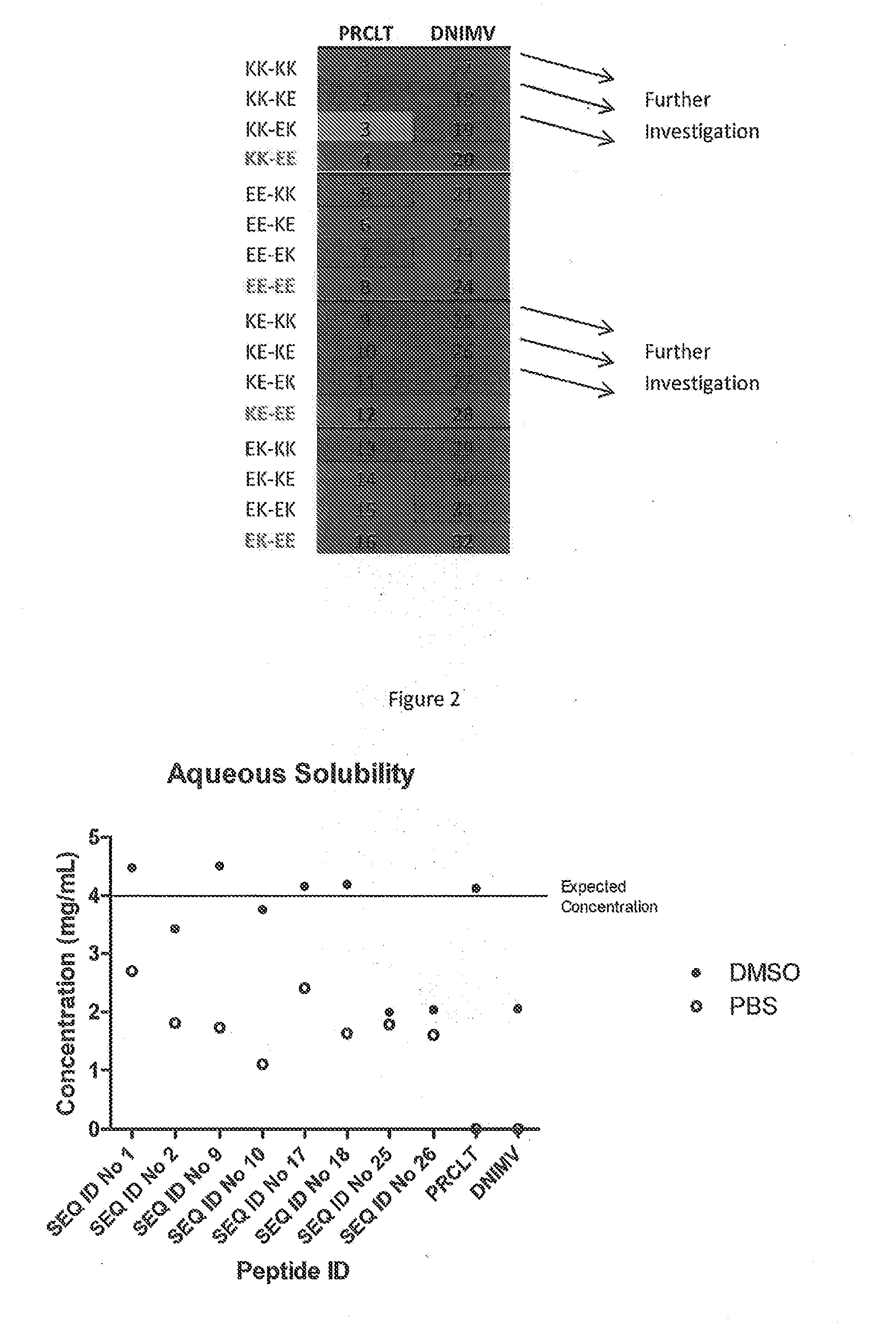
The present invention provides peptides partly derivable from FVIII which are capable of binding to an MHC class II molecule without further antigen processing and being recognised by a factor VIII specific T cell. In particular, the present invention provides peptides comprising the sequence DNIMVTFRNQASRPY or PRCLTRYYSSFVNME with modifications at the N- and C-termini. The present invention also relates to the use of such a peptide for the prevention or suppression of inhibitor antibody formation in haemophilia A and / or acquired haemophilia.
Owner:APITOPE INT
Recombinant oncolytic Newcastle disease virus for expressing human GM-CSF and use thereof
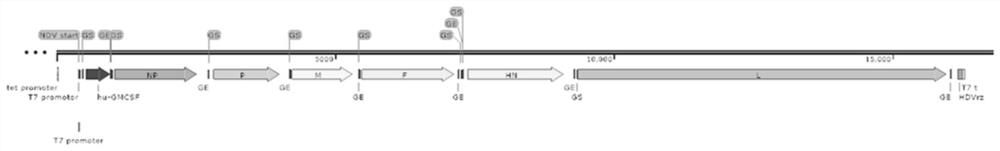
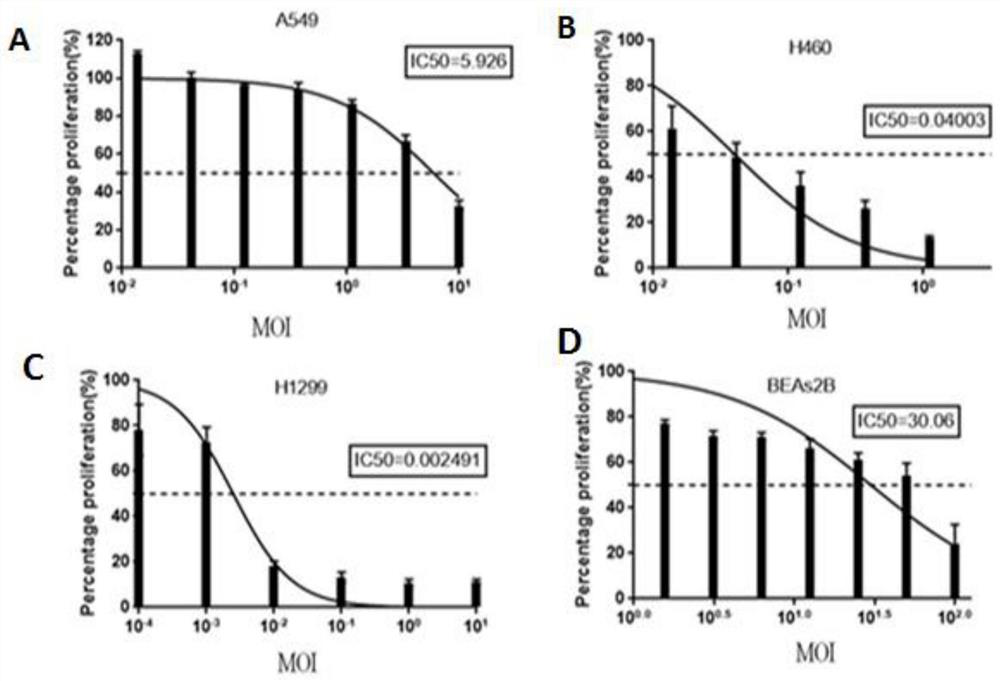
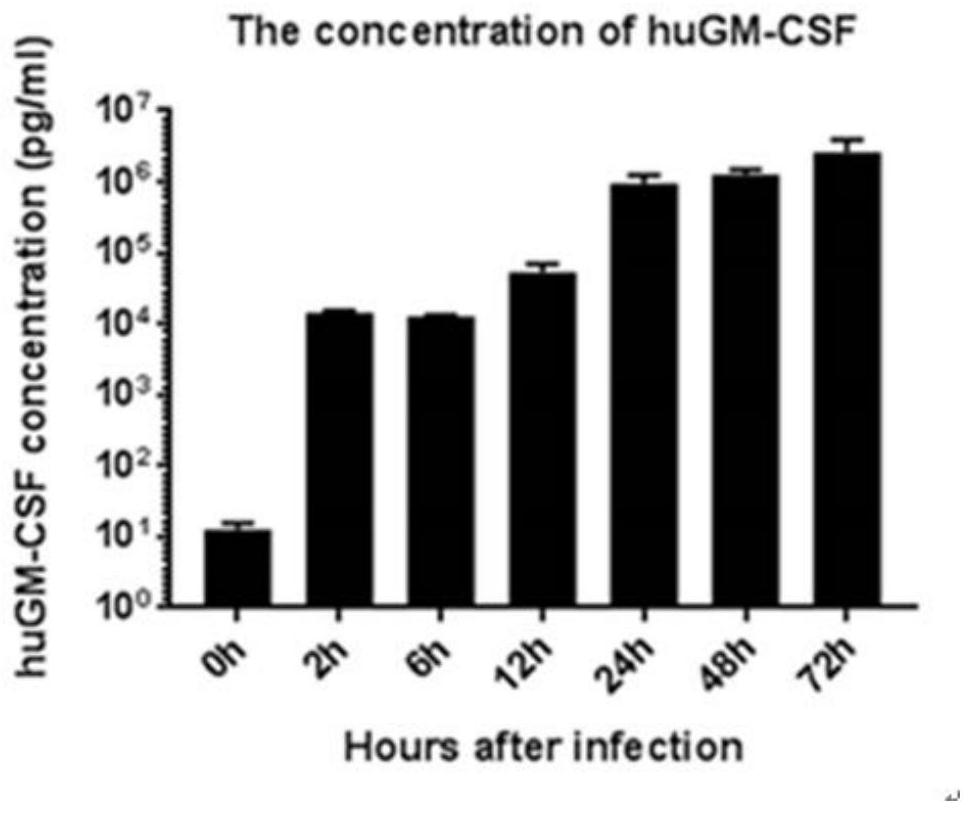
PendingCN113355295APromote differentiation and maturationEasy to processSsRNA viruses negative-sensePeptide/protein ingredientsTherapeutic effectTumor antigen
The invention relates to the field of biological virus construction and particularly discloses a recombinant oncolytic Newcastle disease virus for expressing human GM-CSF. The recombinant oncolytic Newcastle disease virus is obtained by inserting a human GM-CSF coding gene into a full-length genome plasmid of a Newcastle disease virus Italien strain and reviving the virus in vitro. The human GM-CSF gene is as shown in SEQ ID NO.1. A GM-CSF molecule is used for recruiting DC cells from mononuclear cells, activating neutrophil and promoting differentiation and maturation of the DC cells, especially has an important effect on promoting processing and presentation of antigens, combines a characteristic of selective replication of tumors of the oncolytic Newcastle disease virus, directly kills tumor cells, causes inflammation, recruits more DC cells, promotes maturation of the DC cells and presentation of tumor antigens, enhances local and whole-body anti-tumor immune responses of tumors, improves a treatment effect of single or combined use of the oncolytic virus in treating solid tumors, and brings a new treatment thought for tumor immunotherapy.
Owner:FOURTH MILITARY MEDICAL UNIVERSITY
Single-domain antibody for recognizing complex formed by HLA-A2 molecule and ITDQVPFSV short peptide
InactiveCN110872347AHigh affinityHybrid immunoglobulinsImmunoglobulins against cell receptors/antigens/surface-determinantsAntigenAntiendomysial antibodies
The invention discloses a single-domain antibody for recognizing a complex formed by an HLA-A2 molecule and an ITDQVPFSV short peptide. The single-domain antibody has an amino acid sequence shown in SEQ ID No.10 or SEQ ID No.11 or SEQ ID No.12. The invention also discloses a fusion protein obtained by fusing the single-domain antibody and a human-derived Fc protein. Experiments show that the single-domain antibody and fusion protein provided by the invention not only recognize the artificially synthesized HLA-A2 / ITDQVPFSV complex, but also can bind to the HLA-A2 / ITDQVPFSV complex processed bya natural antigen and expressed at the surfaces of tumor cells, and can be further developed into a related tumor treatment product.
Owner:SHENZHEN BEIKE BIOTECH
Tapasin augmentation for enhanced immune response
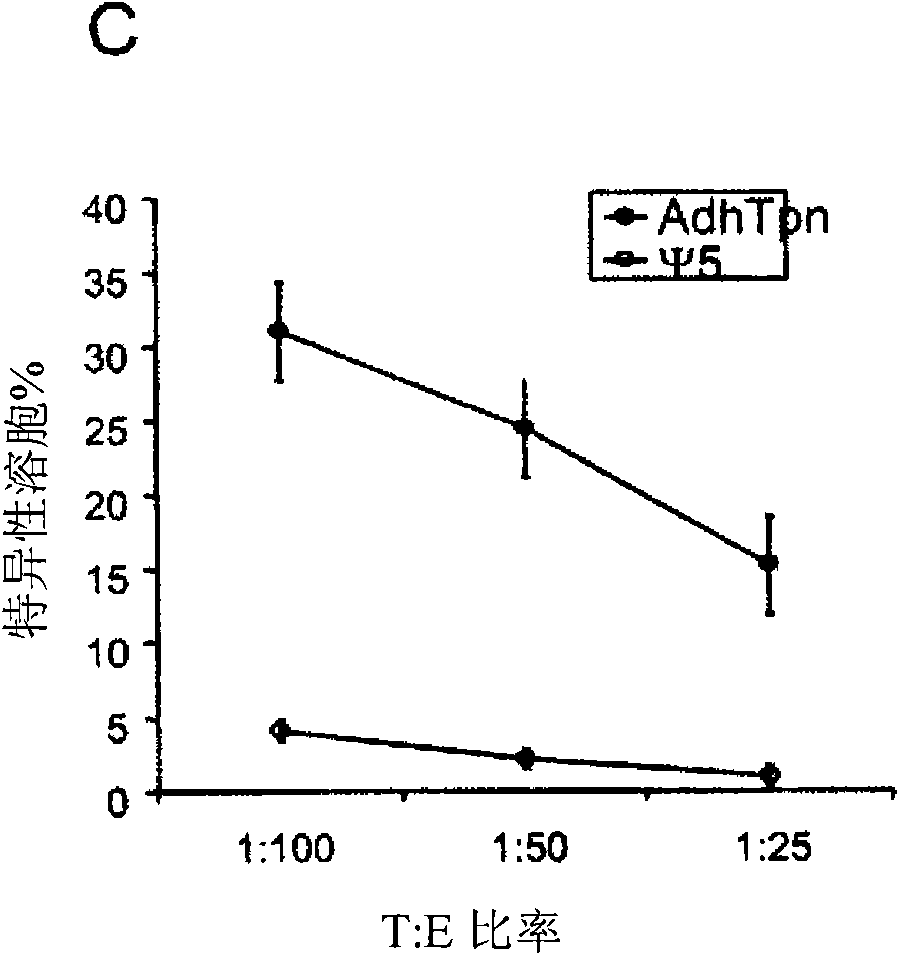
Tapasin (Tpn) is a member of the MHC Class I loading complex and functions to bridge the TAP peptide transporter to MHC Class I molecules. Metastatic human carcinomas express low levels of the antigen processing components (APCs) tapasin and TAP, and display few functional surface MHC Class I molecules. As a result, carcinomas are often unrecognizable by effector cytolytic T cells (CTLs). Tpn alone can enhance survival and immunity of mammals against tumors, but additionally, Tpn and TAP can be used together as components of immunotherapeutic vaccine protocols to eradicate tumors.
Owner:塔皮穆内公司
Pox viridae treatment
InactiveUS20100303894A1Increases MHC Class -antigen presentationImprove responsePowder deliveryPeptide/protein ingredientsAntigenInfected cell
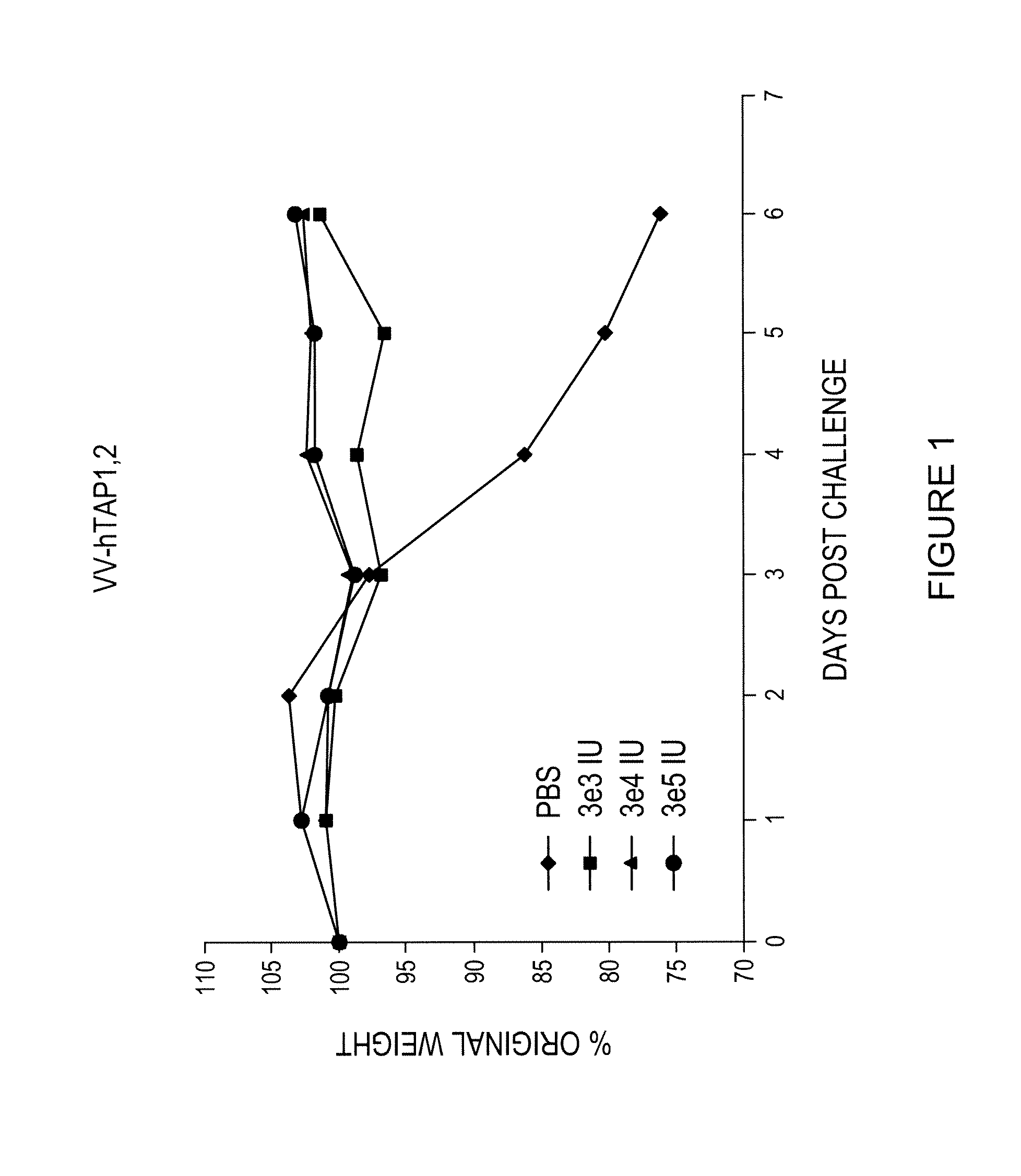
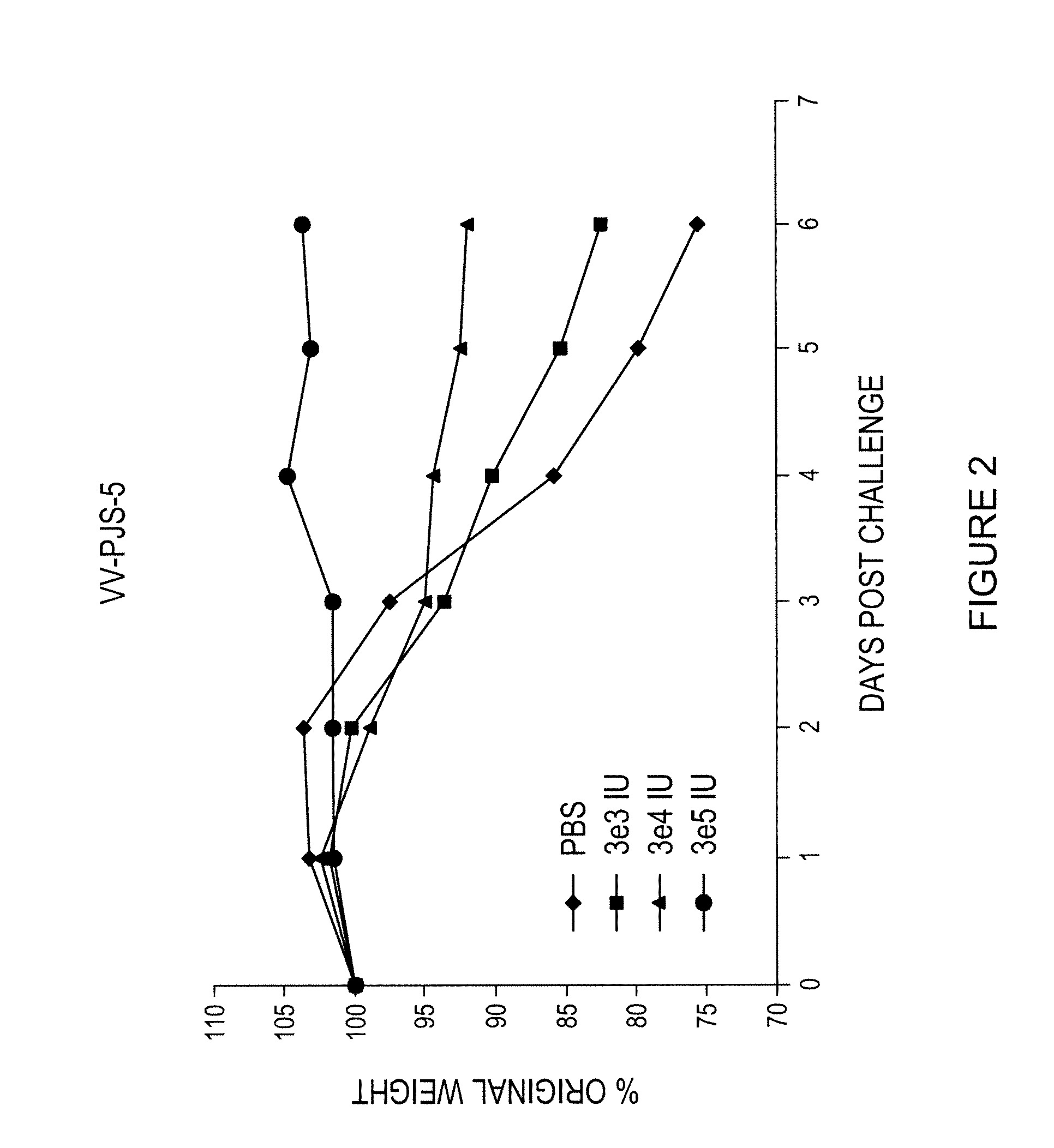
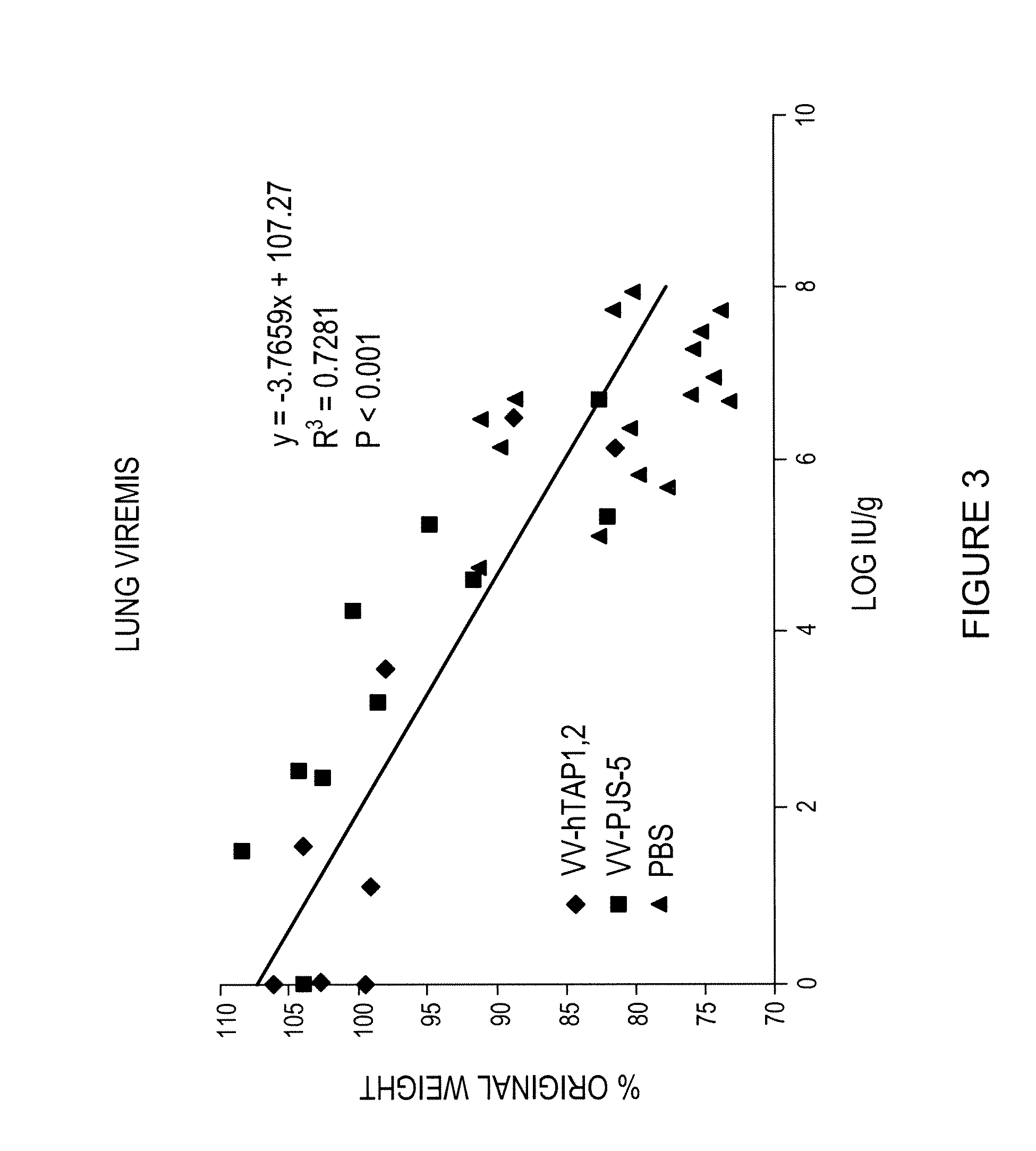
A vaccine composition for combating Pox viridae viral infections in living organisms such as mammals (including humans) comprises TAP-1 and / or TAP-2 to augment the antigen processing capability of infected cells and hence their immunogenicity. The composition may be used alone or, preferably, as an immunogenicity-enhancing adjuvant with a pox antigen-based vaccine, especially in the treatment or prophylaxis of viral infections such as smallpox.
Owner:TAPIMMUNE INC
Screening and validation of human papillomavirus type 16 tripeptide vaccine and construction of tumor animal models that continuously express hpv16 E5, E6, E7
ActiveCN102343103BPrevent proliferationGrowth inhibitionViral antigen ingredientsGenetic material ingredientsAdjuvantTGE VACCINE
The invention discloses screening and verifying of a human papilloma virus (HPV) 16 tripeptide vaccine and a construction scheme of a tumor animal model for continually expressing HPV16 E5, E6 and E7. Polypeptide fragments with high scores are screened with a method for analyzing polypeptide fragments specific to a transporter associated with antigen processing (TAP) in combination with bioinformatics, a tripeptide vaccine core consists of three fragments with strongest immune effects obtained by performing in-vivo and in-vitro tests, and an HPV16 vaccine with preventing and treating functions is constructed in combination with a CPG adjuvant. Simultaneously, a tumor animal model for continually expressing HPV16 E5, E6 and E7 is constructed. The invention is technically characterized in that: by stably integrating HPV16E5 into a TC-1 cell through an adeno-associated virus expression system and injecting the modified TC-1 cell into a C57BL / 6 mouse body, a tumor can be formed successfully.
Owner:马丁
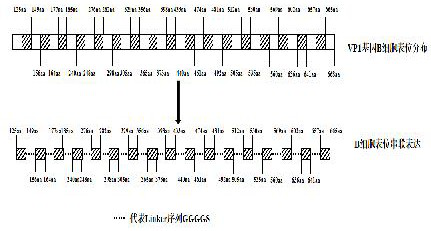
Tandem expression of universal epitopes of feline calicivirus gi and gii strains and establishment of an indirect ELISA method
ActiveCN113801243BImmunogenicHigh sensitivitySsRNA viruses positive-senseAntibody mimetics/scaffoldsFeline calicivirus infectionCellular antigens
The present invention provides a tandem expression of common antigenic epitopes of feline calicivirus GI and GII strain groups and the establishment of an indirect ELISA method. Using a prokaryotic expression vector, multiple B cell antigens of feline calicivirus VP1 protein were expressed in tandem. The expressed recombinant protein was purified and used as a coating antigen for the detection of feline calicivirus antibodies. A parallel comparison with the method of encapsulating whole virus showed that the value of detecting negative and positive sera was highly consistent. The antigen processing of the invention is convenient, the test time is shortened, and the operation steps are simpler. The invention establishes an indirect ELISA method for detecting the antibody level of feline calicivirus in cat serum, has the characteristics of good repeatability and high specificity, and can be used for feline calicivirus serological investigation. Therefore, the feline calicivirus indirect ELISA detection kit based on the tandem expression of the VP1 protein B cell antigen provided by the present invention is very suitable for the detection of large clinical samples, and is suitable for large-scale promotion.
Owner:SHANGHAI VETERINARY RES INST CHINESE ACAD OF AGRI SCI
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com