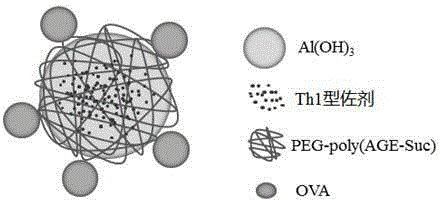
Vaccine vector based on aluminum hydoxide nano-particles
A technology of aluminum hydroxide and nanoparticles, applied in the direction of antibody medical ingredients, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., to achieve the effect of reducing side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Link of PEG-poly(AGE-Suc) to OVA
[0082] Dissolve 1mg of OVA (purchased from Sigma) in 2ml of PBS containing 25mmol / L DTT (dithiothreitol), stir at room temperature for two hours, use PD-10 desalting column (purchased from GE Healthcare) to remove unreacted DTT, etc. For small molecular substances, OVA obtained by breaking the disulfide bond was freeze-dried for later use. Dissolve PEG-poly(AGE-Suc) and linker SPDP (purchased from Pierce Biotechnology) in 1.5ml of PBS-EDTA buffer (the composition is 100 mmol / L Na 3 PO 4 , 150 mmol / L NaCl, 1 mmol / L EDTA, pH=7.5), stirred at room temperature for 45 min, and removed unreacted SPDP etc. with PD-10 desalting cartridge. Dissolve the disulfide-bonded OVA in 1ml of PBS-EDTA, and then add it to the desalted PEG-poly(AGE-Suc) solution (molar ratio PEG-poly(AGE-Suc):OVA=1.2:1) , stirred overnight. Use double-distilled water as the mobile phase, remove small molecular substances and PBS-EDTA solution through a PD-10 desalti...
Embodiment 2
[0085] PEG-poly(AGE-Suc) linked with FITC (fluorescein isothiocyanate)
[0086] Dissolve PEG-poly(AGE-Suc) and FITC (purchased from Sigma) at a molar ratio of 1:1.8 in 10ml Na 2 CO 3 -NaHCO 3 buffer solution (pH=9.6), stirred in the dark for 16 hours, dialyzed in the dark with a 1000Da dialysis bag for 3 days, and freeze-dried.
[0087]
Embodiment 3
[0089] Preparation of Aluminum Hydroxide-OVA-CpG Nanoparticles
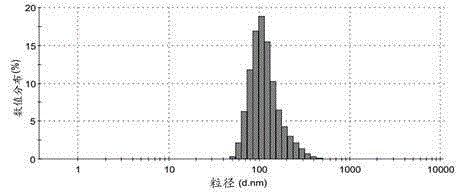
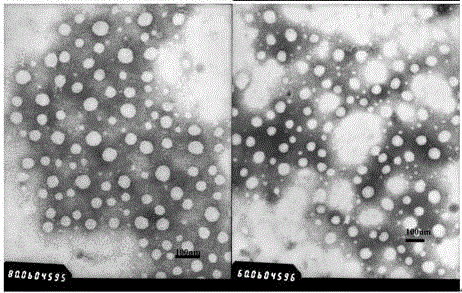
[0090] Add 185 μl 5 mg / ml PEG-poly(AGE-Suc)-OVA solution (Example 1, the same below) (0.925 mg) to 370 μl 100 mmol / L pH=7.6 HEPES buffer (37 μmol), 10 μl 1782 μg / ml CpG- ODN (purchased from Shanghai Sangong) (17.82μg), stir and mix; absorb 555μl 1.67mmol / L Al 2 (SO 4 ) 3 - solution (0.927 μmol), was added to the above mixed solution, stirred for 30 s to obtain aluminum hydroxide-OVA-CpG nanoparticles. The particle size is detected by Malvern Nano-ZS 90 particle size analyzer, the results can be found in figure 2 . Take a small amount of sample, detect with transmission electron microscope, the result sees image 3 .
[0091]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com