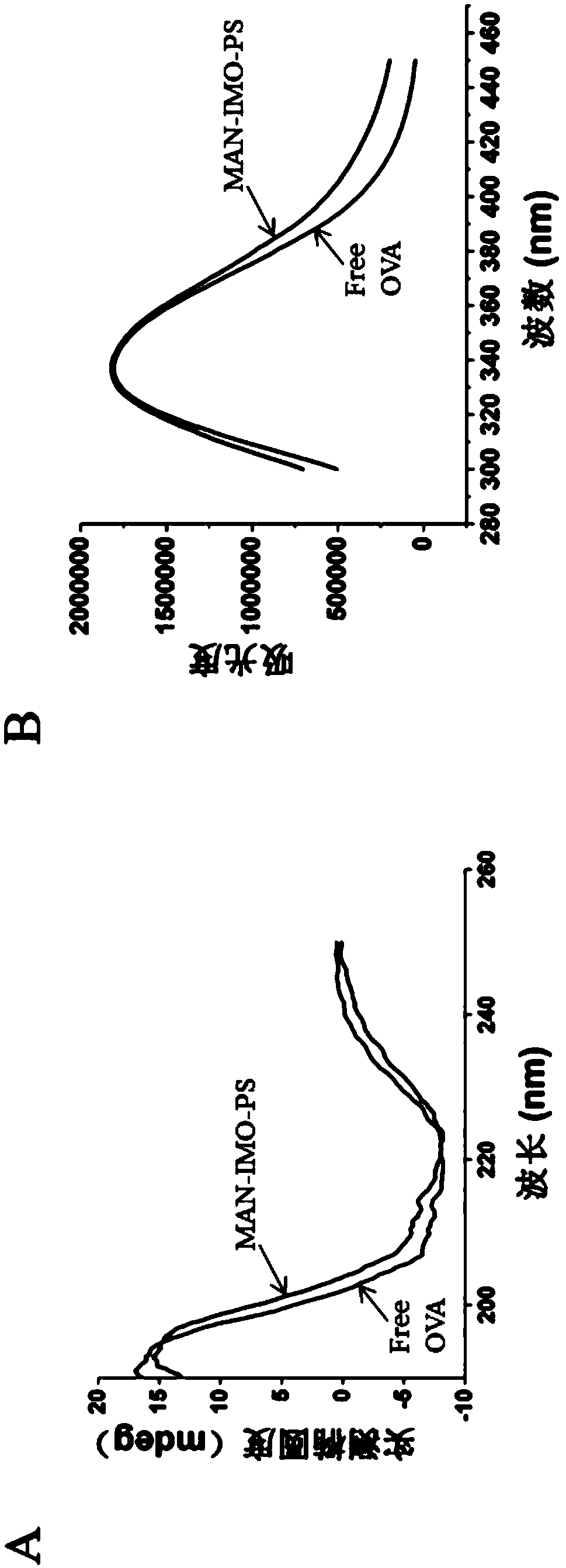
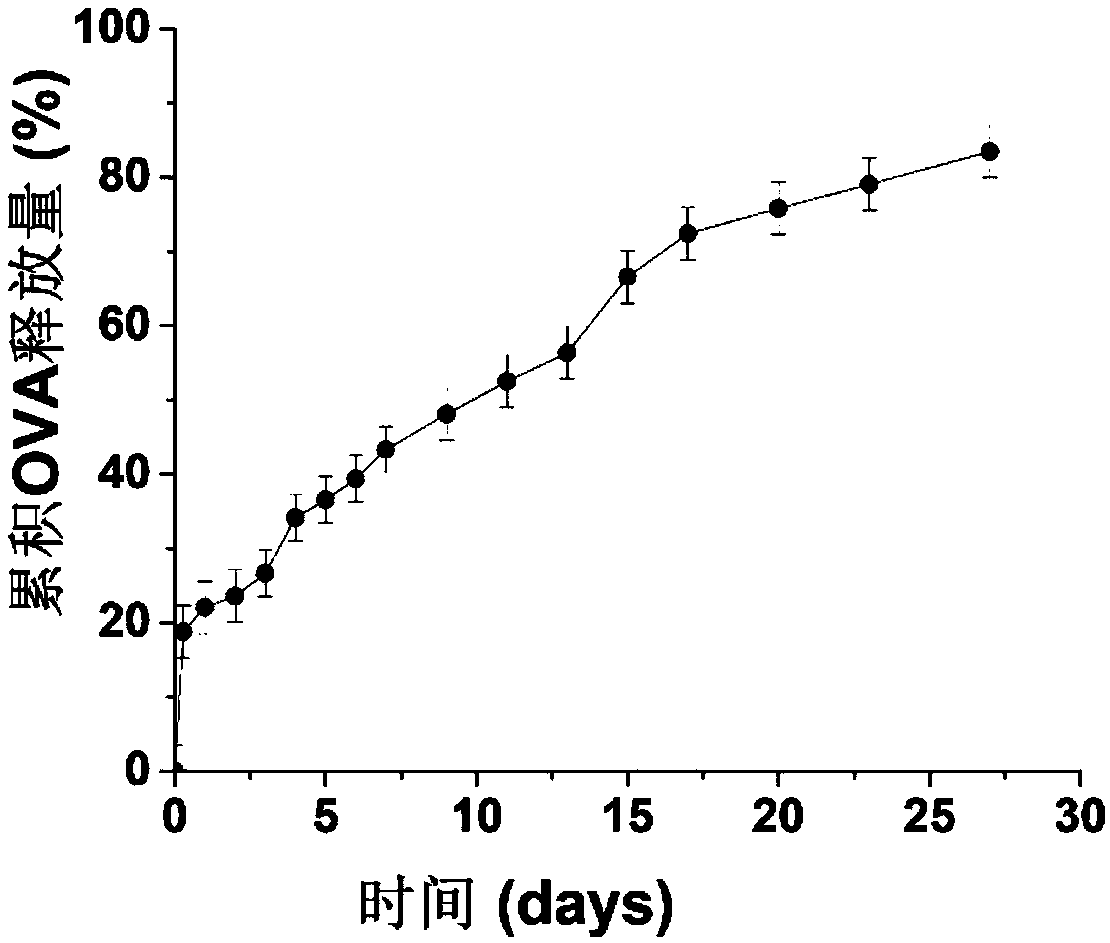
Mannose modified co-loaded antigen and double immuno-agonist phospholipid hybrid polymer vesicle as well as preparation method and application thereof
An agonist and polymer technology, applied in the field of biomedical engineering, can solve the problems of vaccines that cannot produce immune response strength, limit anti-tumor efficiency, etc., and achieve the effect of protecting the body from instability, optimizing the immune effect, and having a small particle size.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The present invention provides a method for preparing phospholipid hybrid polymer vesicles co-carrying antigens and dual immune agonists, comprising steps:
[0039] S1: Dissolve the amphiphilic triblock copolymer PCL-b-PEG-b-PCL and the immune stimulant in an organic solvent; Ultrasonic cell disruptor was used to sonicate for 5 minutes, and the antigen solution was dropped into the sonication process to obtain the primary emulsion;
[0040] Wherein, the mass ratio of the amphiphilic triblock copolymer PCL-b-PEG-b-PCL and the immune stimulant is 20mg:2mg; the molecular weight of the amphiphilic triblock copolymer PCL-b-PEG-b-PCL It is 10000-24000, preferably 16000, wherein the mass percentage of PEG hydrophilic segment is greater than 45%; the amphiphilic triblock copolymer PCL-b-PEG-b-PCL is preferably PCL 4000 -PEG 8000 -PCL 4000 The immune stimulant is one or more of TLR4, TLR7 / 8, TLR1, TLR2, TLR5, TLR6, TLR3 and TLR9; preferably, the TLR7 / 8 agonist is IMQ and / or R...
Embodiment 1
[0052] This example provides a method for preparing phospholipid hybrid polymer vesicles co-loaded with antigens and dual immune agonists, including steps:
[0053] S1: 20 mg amphiphilic triblock copolymer PCL 4000 -PEG 8000 -PCL 4000 and 2mg of TLR7 / 8 agonist IMQ were dissolved in 1mL of dichloromethane; after fully dissolving, in an ice bath, ultrasonicated for 5min with a 3mm probe and an ultrasonic cell disruptor whose power was adjusted to 25% (16W). Inject 200 μL of 10 mg / mL OVA antigen solution to obtain a primary emulsion.
[0054] S2: Under the condition of magnetic stirring at 200rpm, drop the primary emulsion into 10mL of swollen polyvinyl alcohol (PVA) solution with a mass fraction of 2%, and the dropping time is 2min, then wash it with 1mL deionized water. Immediately, the cleaned mixture was ultrasonicated for 10 min with a 5mm probe and an ultrasonic cell disruptor with power adjusted to 30% (22W) in an ice bath to obtain a secondary emulsion.
[0055] S3: S...
Embodiment 2
[0059] This example provides a method for preparing phospholipid hybrid polymer vesicles co-loaded with mannose-targeted modified antigens and dual immune agonists, including steps:
[0060] S1: 20 mg amphiphilic triblock copolymer PCL 4000 -PEG 8000 -PCL 4000 and 2mg of TLR7 / 8 agonist IMQ were dissolved in 1mL of dichloromethane; after fully dissolved, under ice bath conditions, ultrasonic cell disruptor with 3mm probe and power adjusted to 25% (16W) was ultrasonicated for 5min. 200 μL of 10 mg / mL OVA antigen solution was added dropwise to obtain a primary emulsion.
[0061] S2: Under the condition of magnetic stirring at 200rpm, drop the primary emulsion into 10mL of swollen polyvinyl alcohol (PVA) solution with a mass fraction of 2%, and the dropping time is 2min, then wash it with 1mL deionized water. Immediately, the cleaned mixture was ultrasonicated for 10 min with a 5mm probe and an ultrasonic cell disruptor with power adjusted to 30% (22W) in an ice bath to obtain ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com