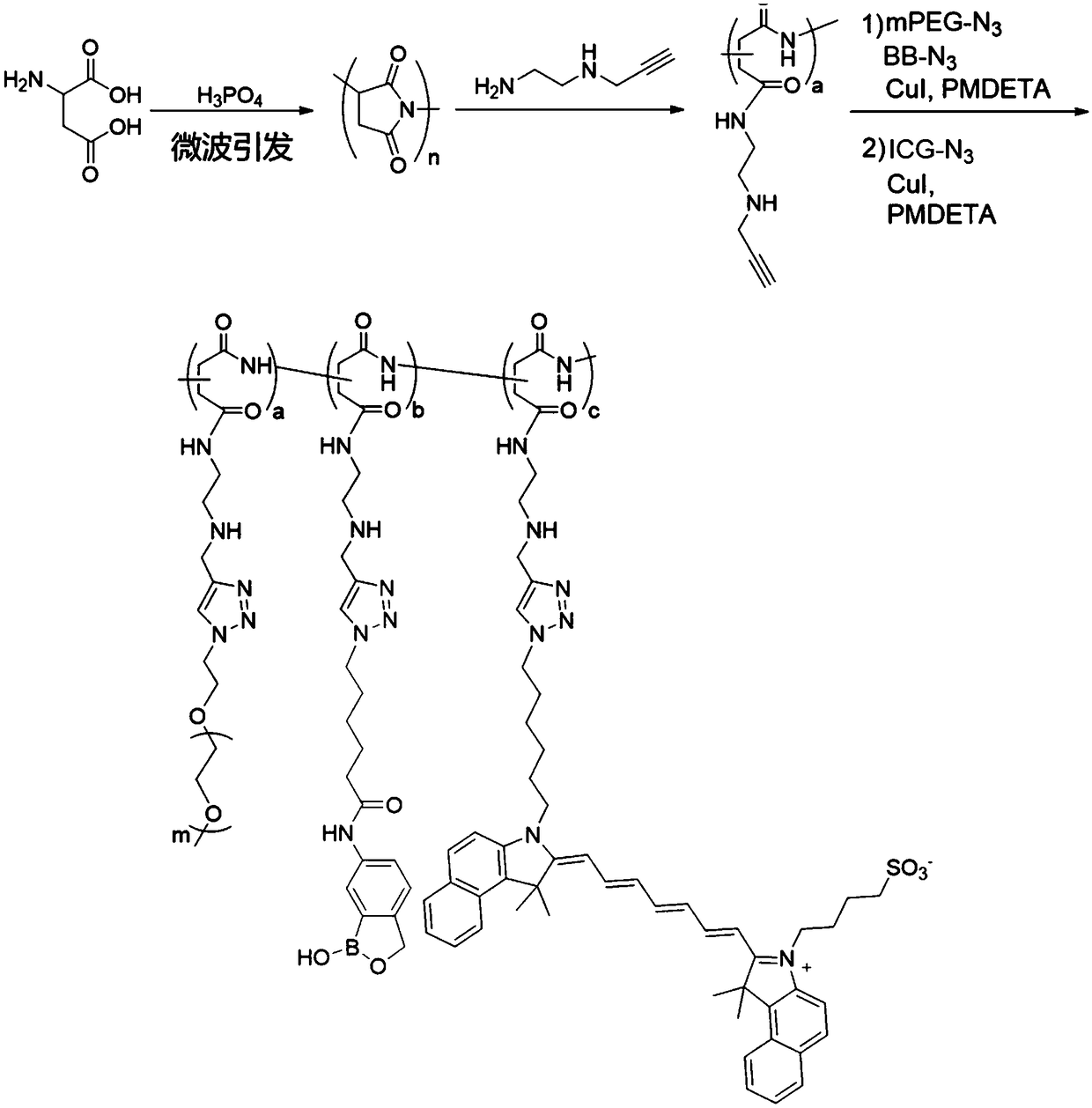
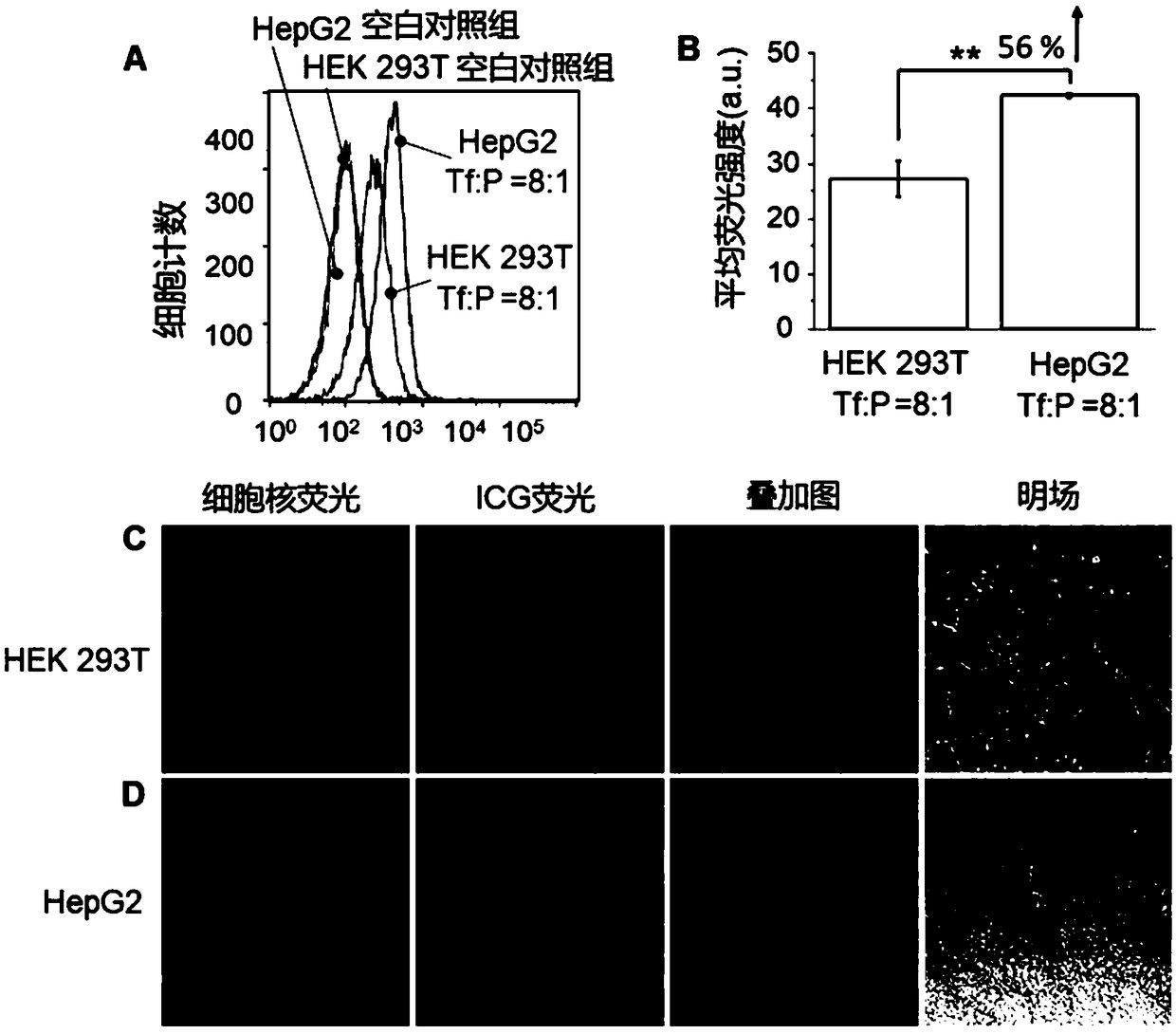
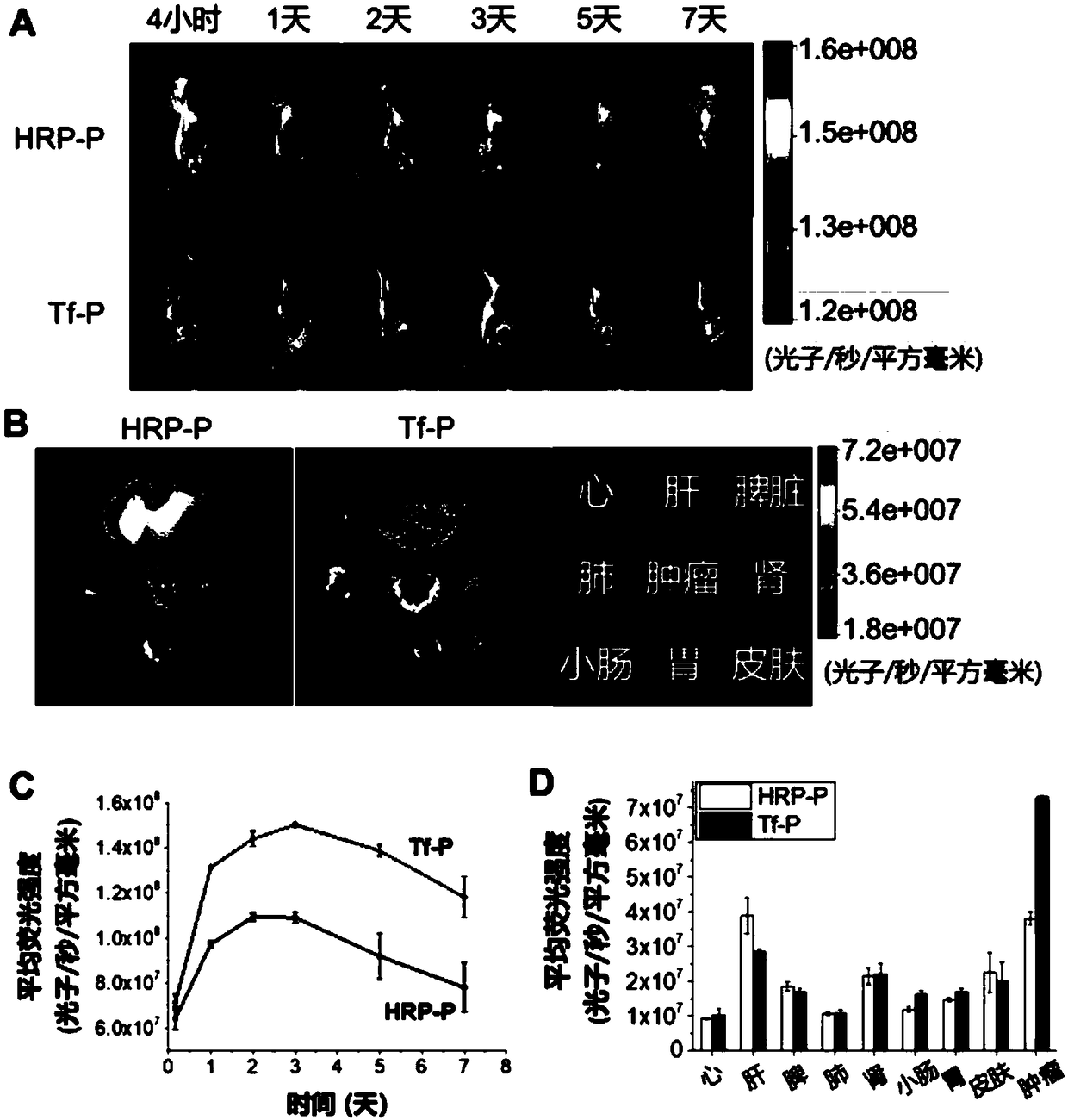
Antibody conjugate with boron ester unit polymers and method for fabricating antibody conjugate
An antibody conjugate and polymer technology, applied in the field of biomedicine, can solve the problems of inability to achieve targeted therapeutic effect, reduced therapeutic effect, and reduced antibody-antigen binding rate, achieves excellent tumor targeting effect, and has a clear preparation process. Concise, excellent biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Preparation of polymethacrylic acid (PMAA) nanogel microsphere-transferrin (Tf) conjugate
[0024] Step 1: Preparation of PMAA Microspheres
[0025] Monomer methacrylic acid (MAA), crosslinking agent N,N-methylenebisacrylamide (MBA) and initiator azobisisobutyronitrile (AIBN) were added in acetonitrile in a certain proportion and mixed evenly, heated to 95℃, The solvent was refluxed for 2 hours, the solvent and unreacted monomer were removed by centrifugation, washed three times with ethanol and deionized water, and freeze-dried for 24 hours;
[0026] Step 2: Surface modification of PMAA microspheres with 2-(hydroxymethyl)phenylboronic acid cyclic monoester units
[0027] A certain amount of PMAA nanogel microspheres was dispersed in phosphate buffer solution (pH=7.4, the same below), and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) was added. ) and N-hydroxysuccinimide (NHS), reacted at room temperature for 1 hour to activate the carbo...
Embodiment 2
[0030] Example 2: Preparation of PMAA nanogel microsphere-vascular endothelial growth factor receptor antibody (Anti-VEGF Receptorantibody) conjugate
[0031] Disperse PMAA-BB microspheres (the preparation method is the same as in Example 1) in phosphate buffer solution, add a certain amount of phosphate buffer solution of vascular endothelial growth factor receptor antibody, mix well and let stand for 10 minutes until it fully reacts. for subsequent applications.
Embodiment 3
[0032] Example 3: Preparation of polyaspartic acid-loaded micelle-Tf conjugate
[0033] Step 1: Preparation and Alkynylation of Polyaspartic Acid
[0034] Mix L-aspartic acid powder, 85% phosphoric acid (H 3 PO 4 ), sulfolane and mesitylene were mixed, after microwave reaction, washed with water and ethanol for many times, and vacuum dried to obtain polysuccinimide with 2-[2-(2-propynyloxy)ethoxyl ]Ethylamine was dissolved in dimethylformamide (DMF), reacted at room temperature for 12 hours, after several times of DMF dissolution-diethyl ether precipitation and centrifugation, and vacuum-dried to obtain alkynyl-modified polyaspartic acid;
[0035] Step 2: Polyaspartic acid grafted polyethylene glycol, 2-(hydroxymethyl)phenylboronic acid cyclic monoester and drug molecule
[0036] The alkynyl-modified polyaspartic acid was dissolved in DMF, and the DMF-dissolved azide polyethylene glycol monomethyl ether (mPEG-N 3 ) and 2-(hydroxymethyl)benzeneboronic acid azide monoester (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com