Varicella-zoster virus vaccine and application thereof
A herpes zoster virus and vaccine technology, applied in the field of vaccines, can solve problems such as research that has not been clinically applied
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
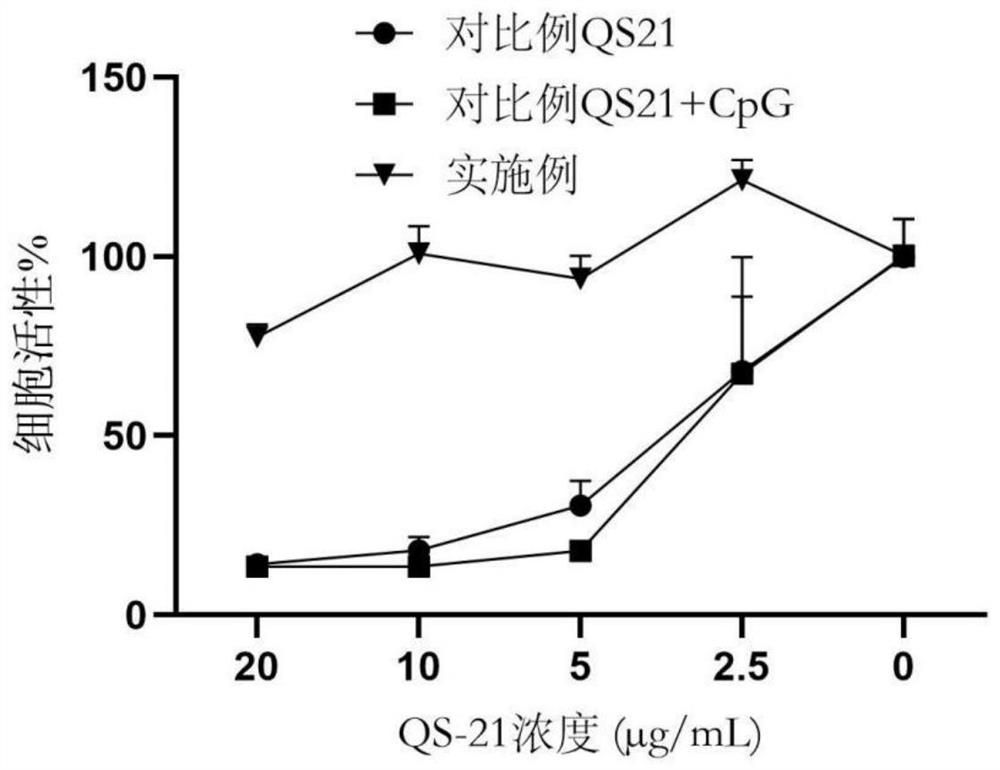
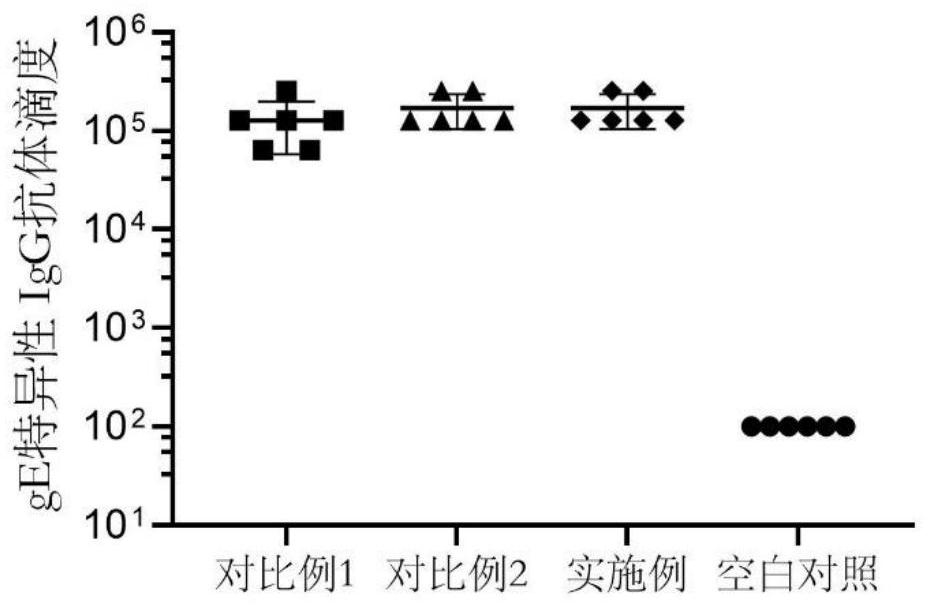
Examples
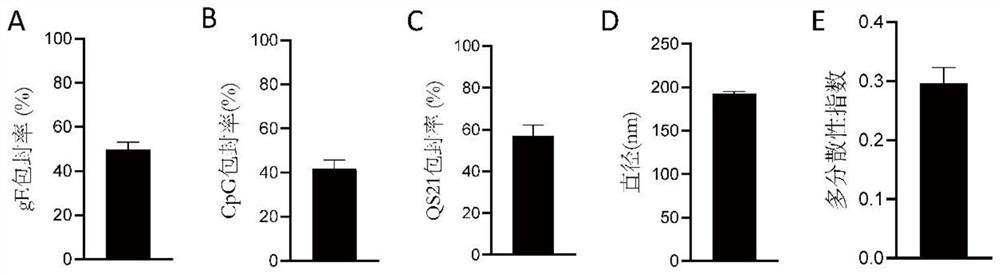
experiment example 1
[0049] Experimental example 1, gE concentration
[0050] EXAMPLES Vaccines were lysed overnight at room temperature in 0.1 M sodium hydroxide and 0.1% (w / v) sodium dodecyl sulfate buffer. Use BCA colorimetric protein detection kit (Shanghai Biyuntian Biotechnology Co., Ltd.) to detect the gE concentration and calculate the protein loading efficiency.
experiment example 2
[0051] Experimental example 2, nucleic acid concentration
[0052] EXAMPLES Vaccines were lysed overnight at room temperature in 0.1 M sodium hydroxide and 0.1% (w / v) sodium dodecyl sulfate buffer. The nucleic acid concentration was detected and the nucleic acid loading efficiency was calculated using the nucleic acid detection kit Quant-iT OliGreen ssDNA Regent Kit (purchased from Thermo Fisher).
experiment example 3、Q
[0053] Experimental example 3, QS21 concentration
[0054] EXAMPLES Vaccines were lysed overnight at room temperature in 0.1 M sodium hydroxide and 0.1% (w / v) sodium dodecyl sulfate buffer. Using free QS21 as the standard, high performance liquid chromatography (HPLC, purchased from Waters Company) with a 4.6×250 mm C18 column (purchased from Waters Company) was used to measure the content of QS21 encapsulated in the examples and calculate the QS21 loading efficiency.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| polydispersity index | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com