Liposome preparation for tumor therapeutic vaccine and preparation method thereof
A technology of liposome preparation and tumor treatment, which is applied in the direction of liposome delivery, anti-tumor drugs, medical preparations of non-active ingredients, etc., and can solve the problem of inability to produce therapeutic vaccine protein antigen cross-presentation and inability to effectively protect Antigen etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
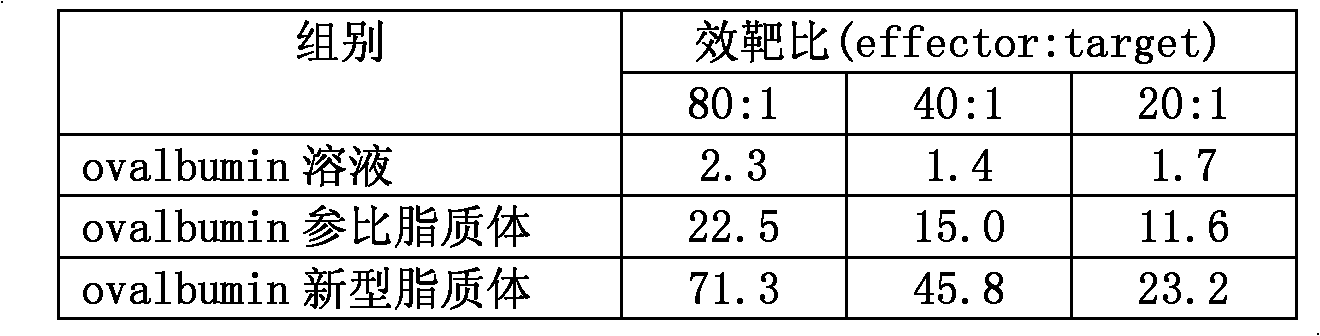
[0027] Dissolve 20 mg / mL chicken ovalbumin (ovalbumin) solution with 0.3% sodium carbonate solution, adjust isotonicity with sodium chloride, adopt a lipid prescription with a molar ratio of lecithin (EPC) / cholesterol (cholesterol) 1 / 1, pass through ethanol Prepare liposomes by injection method, dissolve 20mg EPC and 10mg cholesterol in 1mL ethanol, filter the ethanol solution with a microporous membrane, and set aside. Add 14mL of 20mg / mL ovalbumin in 0.3% sodium carbonate solution into a 50mL flask containing a stirring bar, and then rapidly inject 1mL of lipid ethanol solution into the liposome-forming suspension under high-speed stirring. Ethanol was removed by dialysis, and then the prepared liposomes were ultrasonicated so that the average particle size was 500 nanometers.
[0028] Separation of liposome and free protein by using a molecular sieve gel column, 10% Triton solution to disrupt the liposome membrane, and quantify the concentration of ovalbumin in it by HPLC, ...
Embodiment 2
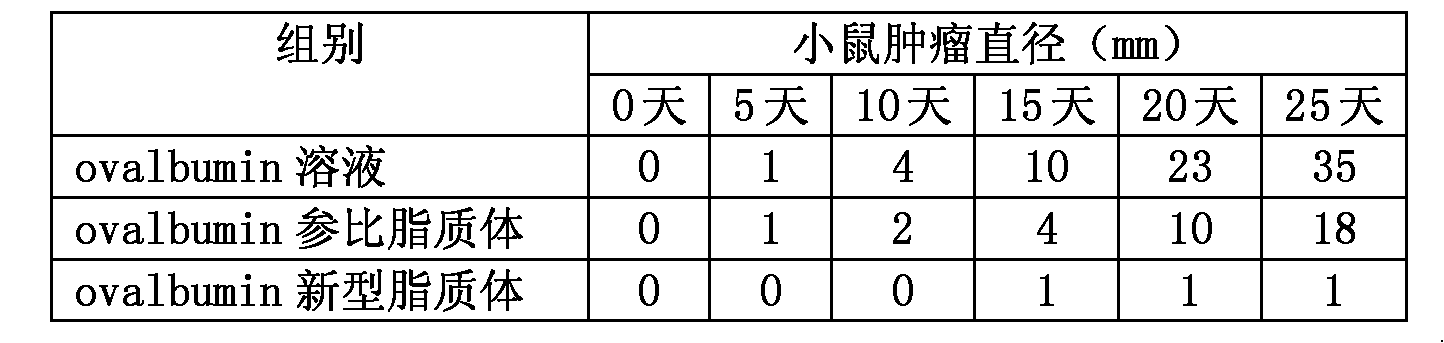
[0034]Prepare liposomes by freeze-drying, dissolve ovalbumin with 0.9% sodium bicarbonate to form a 2 mg / mL protein solution, adjust isotonicity with sodium chloride, dissolve 40 mg DPPC and 10 mg cholesterol in chloroform solution, and place in an eggplant-shaped bottle In the process, chloroform was removed under low pressure on a rotary evaporator, 1 mL of ovalbumin solution was added to hydrate, liquid nitrogen was frozen, freeze-dried overnight, and rehydrated to obtain a liposome suspension. The prepared liposomes were repeatedly frozen and thawed and ultrasonicated, and then passed through 1000nm, 400nm, 200nm, 100nm membranes successively to make the average particle size about 100nm.
[0035] The liposome and free protein were separated by molecular sieve gel column, the liposome membrane was broken with 10% Triton solution, and the concentration of ovalbumin was quantified by HPLC to determine the components of the prepared liposome. The sodium bicarbonate concentrat...
Embodiment 3
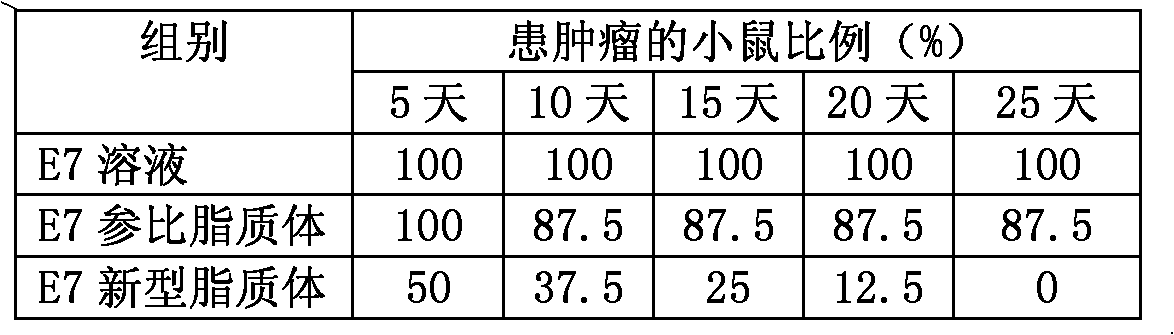
[0041] Prepare liposomes by film dispersion method, dissolve 5mg DPPC, 0.6mg cholesterol and 0.4mg DOTAP in chloroform solution, put them into an eggplant-shaped bottle, put them in a water bath at 30°C, remove the chloroform by rotary evaporation to form a film, and keep vacuum for 1 hour To remove the solvent, dissolve the ovalbumin protein in 3% sodium bicarbonate to a 10mg / mL solution, add 1mL ovalbumin solution to the eggplant-shaped bottle, ultrasonically oscillate, and place at room temperature for 2h to form a translucent lipoplex suspension . The obtained liposomes are subjected to high-pressure milk homogenization, so that the average particle size thereof is about 200 nm.
[0042] Utilize ultracentrifugation to separate liposome and free protein, 10% Triton solution breaks liposome membrane, and HPLC quantifies ovalbumin concentration therein, so as to measure the component of prepared liposome. The sodium bicarbonate concentration in the final liposome is 3%, the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com