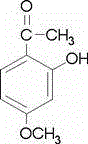
Paeonol niosome emulsifiable paste for external use and preparation method thereof
A technology of paeonol and capsule cream, applied in skin diseases, capsule delivery, ointment delivery, etc., can solve the problems of dermatitis and eczema without specific treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Embodiment 1 paeonol vesicular emulsifiable cream, it consists of:
[0018] Paeonol 20mg, Span6080mg, cholesterol 20mg, dicetyl phosphate 20mg, water for injection 60mL, absolute ethanol 5mL, emulsification time 3h. The vesicle encapsulation rate of paeonol nonionic surfactant obtained by this method was 46.66%, and the drug loading was 6.67%;
[0019] Weigh 5g of glyceryl monostearate, 100g of stearyl alcohol, and 100g of white petrolatum, mix and heat to 80°C to melt into an oil phase; in addition, weigh 20g of Tween-80, 0.5g of disodium hydrogen phosphate, 0.5g of citric acid, Propylene glycol 100g, ethyl p-hydroxybenzoate 3g, purified water, mix and dissolve, heat to 80°C as the water phase, slowly add the water phase to the oil phase, the stirring speed is 800r / min, the emulsification temperature is 80°C, emulsification After 20 minutes, when it cools to 45°C, add paeonol vesicle suspension at the same temperature, continue to stir until the temperature drops to 2...
Embodiment 2
[0020] Embodiment 2 paeonol vesicular emulsifiable cream, it consists of:
[0021] Paeonol 25mg, Span6080mg, cholesterol 20mg, dicetyl phosphate 10mg, water for injection 100mL, absolute ethanol 5mL, emulsification time 2h, the vesicle encapsulation rate of paeonol nonionic surfactant obtained by this method is 46.90% , drug loading 8.69%;
[0022] Weigh 5g of glyceryl monostearate, 120g of stearyl alcohol, and 100g of white petrolatum, mix and heat to 80°C to melt into an oil phase; in addition, weigh 20g of Tween-8020g, 0.5g of disodium hydrogen phosphate, 0.5g of citric acid, Propylene glycol 100g, ethyl p-hydroxybenzoate 3g, purified water, mix and dissolve, heat to 80°C as the water phase, slowly add the water phase to the oil phase, the stirring speed is 800r / min, the emulsification temperature is 60°C, emulsification After 20 minutes, when it cools to 45°C, add paeonol vesicle suspension at the same temperature, continue to stir until the temperature drops to 25°C, tes...
Embodiment 3
[0023] Embodiment 3 Paeonol nonionic surfactant vesicles, which consist of:
[0024] Paeonol 25mg, Span6090mg, cholesterol 30mg, dicetyl phosphate 10mg, water for injection 60mL, absolute ethanol 8mL, emulsification time 3h, the encapsulation rate of paeonol nonionic surfactant vesicle obtained by this method is 63.26 %, drug loading 10.2%;
[0025] Weigh 5g of glyceryl monostearate, 120g of stearyl alcohol, and 100g of white petrolatum, mix and heat to 70°C to melt into an oil phase; in addition, weigh 20g of Tween-8020g, 0.5g of disodium hydrogen phosphate, 0.5g of citric acid, Propylene glycol 100g, ethyl p-hydroxybenzoate 3g, purified water, mix and dissolve, heat to 70°C as the water phase, slowly add the water phase to the oil phase, the stirring speed is 800r / min, the emulsification temperature is 60°C, emulsification After 20 minutes, when it cools to 45°C, add paeonol vesicle suspension at the same temperature, continue to stir until the temperature drops to 25°C, te...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com