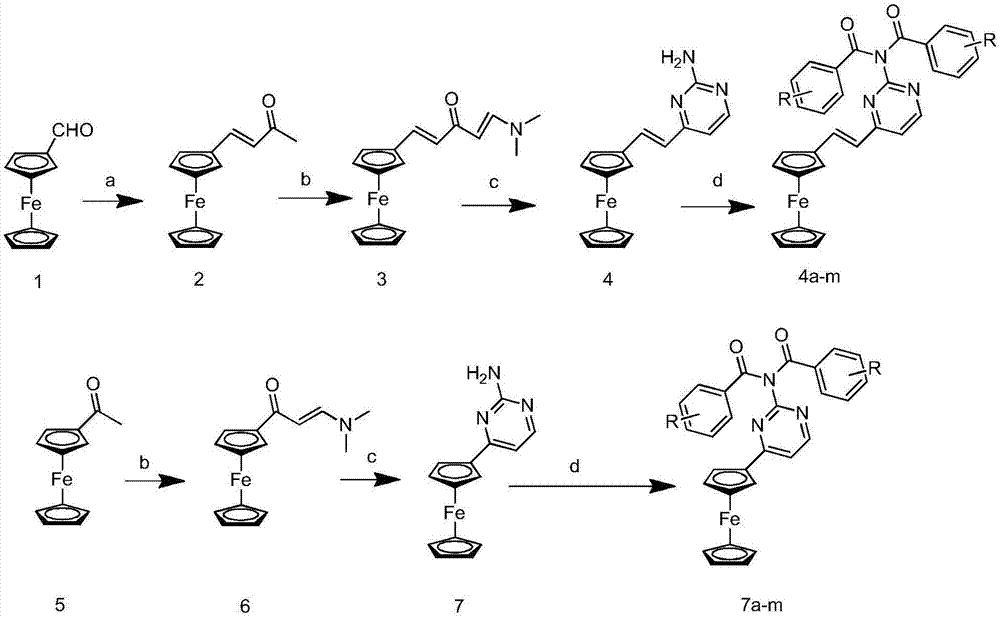
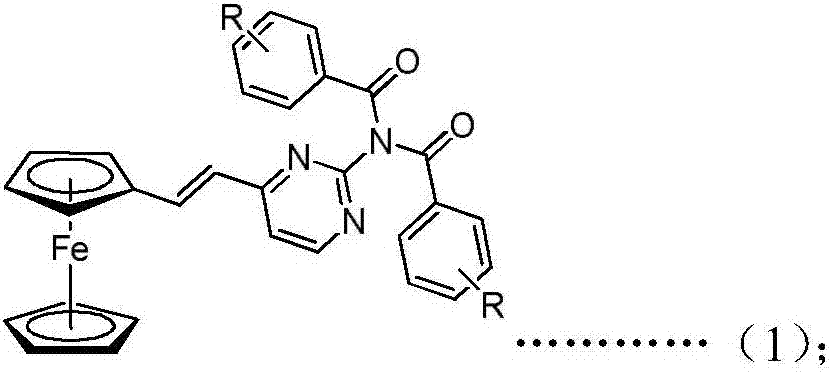
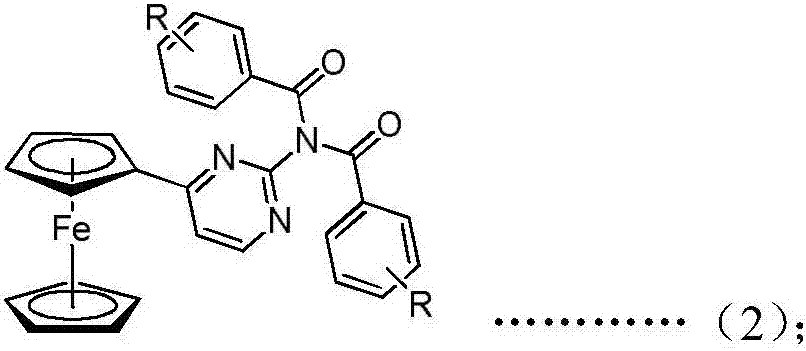
Ferrocenyl amide derivative containing pyrimidine ring and preparation method and appliation thereof
A ferrocene amide and pyrimidine ring technology, applied in the field of ferrocene amide derivatives and their preparation, can solve the problems of drug resistance, increase the risk of blood clots in the lungs, etc., and achieve the effect of inhibiting growth
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Preparation of (E)-4-chloro-N-(4-chlorobenzyl)-N-(4-ferrocenylvinyl)pyrimidin-2-yl)benzamide (4a)
[0033]
[0034] a. Weigh formyl ferrocene (1) (4.8g, 20mmol) in a 250mL single-necked flask, add acetone (100mL), then dropwise add sodium hydroxide solution (2mL, 2mol / L), stir at room temperature, TLC Detection, stop the reaction when the formylferrocene is consumed, add water until the precipitation is complete, filter with suction, dry, and separate by column chromatography (eluent is ethyl acetate / petroleum ether=1 / 2, v / v), 4.22 g of the intermediate (2) methyl ferrocene vinyl ketone was obtained. Deep red solid, yield 83.7%, melting point: 125-127°C.
[0035] 1 H NMR (CDCl 3 ):δ7.42(d,J=16.0Hz,1H),6.34(d,J=16.0Hz,1H),4.50(s,2H),4.44(s,2H),4.15(s,5H),2.29 (s,3H).MS(ESI):255.1(C 14 h 14 FeO,[M+H] + ).
[0036] b. Weigh the intermediate (2) (254mg, 1mmol) and dissolve it in 3mL dimethylformamide, add 595mg of N,N-dimethylformamide dimethyl acetal,...
Embodiment 2
[0043] Example 2: (E)-4-methoxy-N-(4-methoxybenzoyl)-N-(4-ferrocenylvinyl)pyrimidin-2-yl)benzamide (4b ) preparation
[0044]
[0045] The preparation method of this example is the same as that of Example 1, except that in step c, 4-methoxybenzoyl chloride is used instead of 4-chlorobenzoyl chloride to obtain the target product (4b) (E)-4-methoxy- N-(4-methoxybenzoyl)-N-(4-ferrocenylvinyl)pyrimidin-2-yl)benzamide. Yield: 77%, dark red solid, melting point: 91-93°C.
[0046] 1 H NMR (CDCl 3 ): δ8.48(d, J=5.1Hz, 1H), 7.78(d, J=7.4Hz, 4H), 7.43(d, J=15.6Hz, 1H), 7.38(t, J=8.7Hz, 4H ),6.92(d,J=5.1Hz,1H),6.45(d,J=15.6Hz,1H),4.47(s,2H),4.41(s,2H),4.11(s,5H). 13 C NMR (151MHz, CDCl 3 ): δ174.88, 166.85, 165.72, 163.49, 161.24, 141.93, 134.27, 129.65, 124.16, 117.36, 116.53, 82.79, 73.29, 72.18, 70.87, 58.07, 32.29. 32 h 27 FeN 3 o 4 ,[M+H] + ).
Embodiment 3
[0047] Example 3: (E)-4-methyl-N-(4-methylbenzoyl)-N-(4-ferrocenylvinyl)pyrimidin-2-yl)benzamide (4c) preparation
[0048]
[0049] The preparation method of this example is the same as that of Example 1, except that in step c, 4-methylbenzoyl chloride is used instead of 4-chlorobenzoyl chloride to obtain the target product (4c) (E)-4-methyl-N- (4-methylbenzoyl)-N-(4-ferrocenylvinyl)pyrimidin-2-yl)benzamide. Yield: 77%, dark red solid, melting point: 35-37°C.
[0050] 1 H NMR (CDCl 3 ): δ8.50(d, J=5.2Hz, 1H), 7.77(d, J=8.0Hz, 4H), 7.44(d, J=15.6Hz, 1H), 7.18(d, J=8.0Hz, 4H ),6.88(d,J=5.2Hz,1H),6.44(d,J=15.6Hz,1H),4.46(s,2H),4.38(s,2H),4.08(s,5H),2.34(s ,6H). 13 C NMR (151MHz, CDCl 3 ): δ175.38, 166.78, 163.28, 161.26, 145.96, 141.94, 134.62, 132.07, 131.92, 124.03, 117.50, 82.78, 73.29, 72.17, 70.87, 32.34, 24.30.MS(ESI): 542. 32 h 27 FeN 3 o 2 ,[M+H] + ).
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com