Preparation method for ulinastatin freeze-dried powder preparation
A technology of dry powder preparation and ulinastatin, which is applied in the field of preparation of ulinastatin freeze-dried powder preparation, which can solve the problem of affecting the stability of the active ingredient ulinastatin liquid, activated carbon entering human blood, and inability of ulinastatin Protection and other issues, to achieve the effect of facilitating industrial production, increasing control, and improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
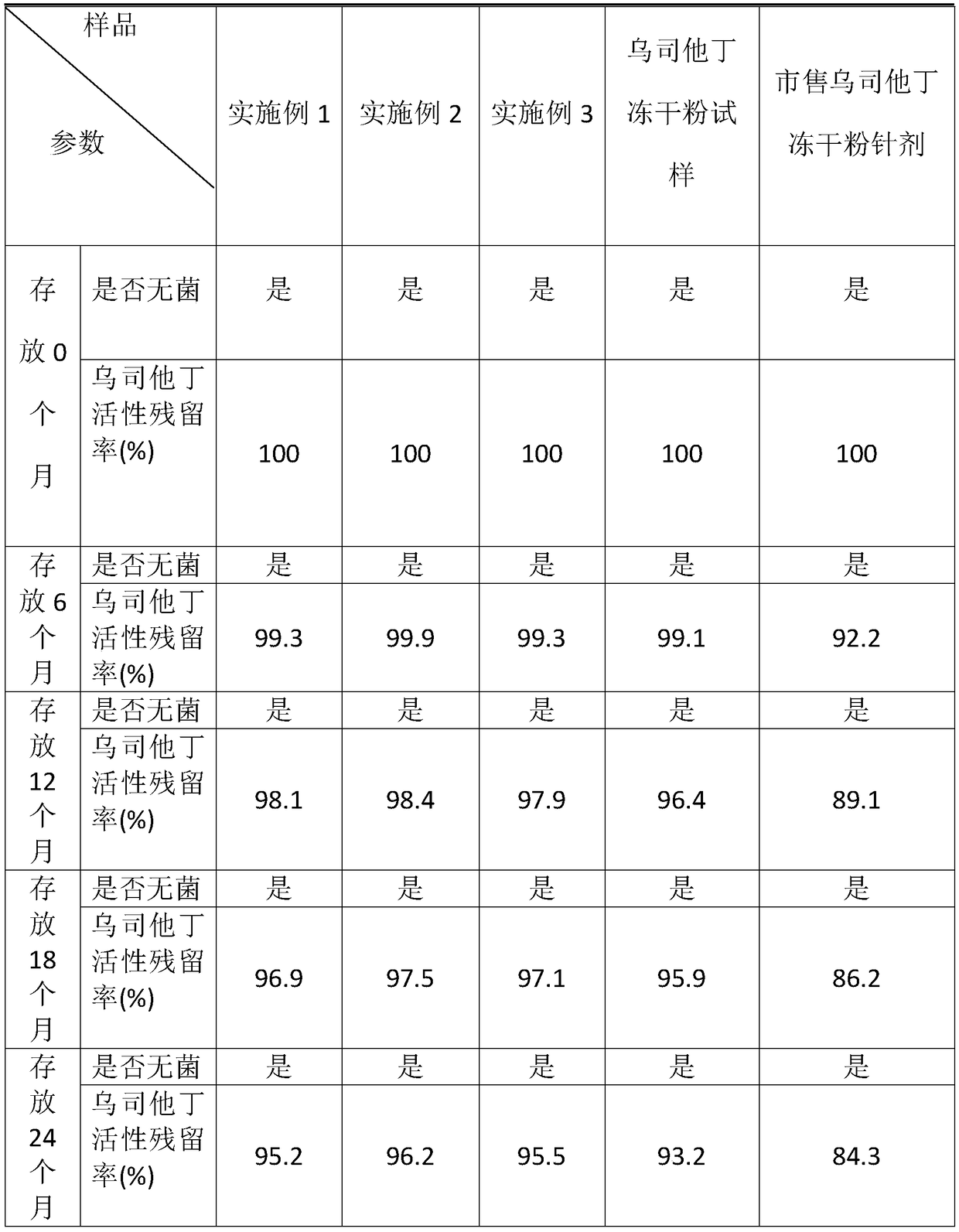
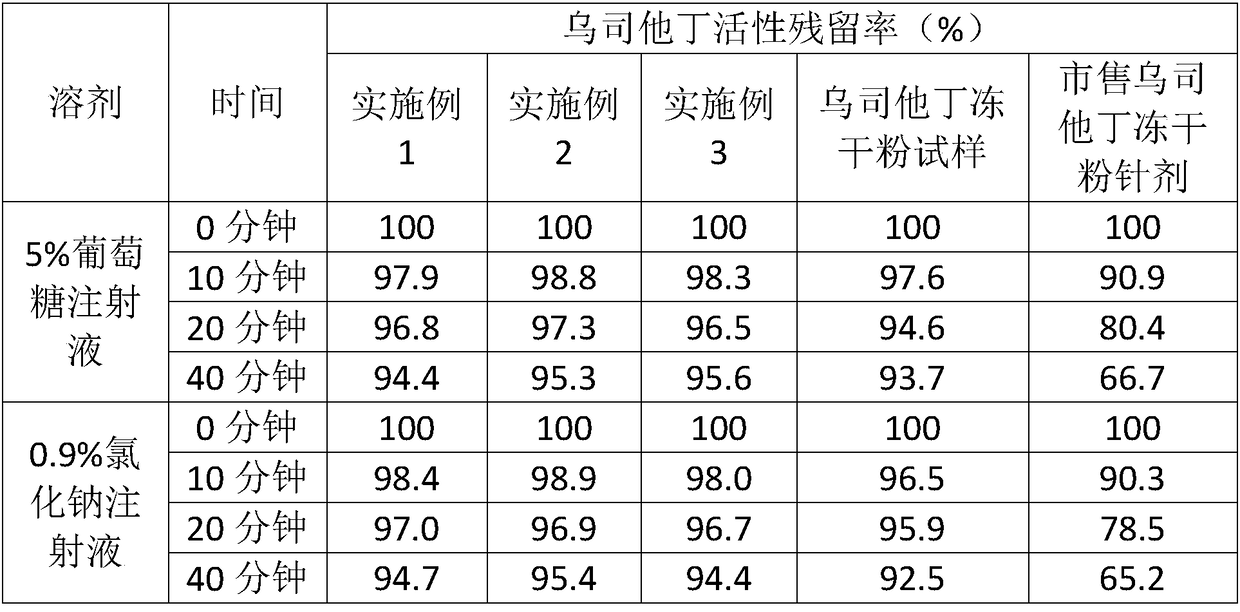
Embodiment 1
[0037] A kind of preparation method of ulinastatin freeze-dried powder preparation, the formula of ulinastatin freeze-dried powder preparation is, every 1000: ulinastatin 25 million units, mannitol 5g, sodium chloride 0.5g, diphosphate Sodium hydrogen 0.5g, disodium hydrogen phosphate 0.5g;
[0038] The preparation method of described ulinastatin freeze-dried powder preparation comprises the following steps:
[0039] (1) Weigh disodium hydrogen phosphate and sodium dihydrogen phosphate in the liquid mixing tank according to the dosage of the formula, stir with water for injection at room temperature until dissolved, and this solution is an auxiliary material solution; dissolve the amount of the above formula with water for injection at room temperature Ulinastatin, and mixed with the above-mentioned adjuvant solution, then add mannitol, sodium chloride, to obtain the medicinal solution A, all the medicinal solutions involved in the following steps (2)-(5) are kept at room temper...
Embodiment 2
[0050] A preparation method of ulinastatin freeze-dried powder preparation, the formula of ulinastatin freeze-dried powder preparation is, per 1000: ulinastatin 50 million units, mannitol 10g, sodium chloride 1g, dihydrogen phosphate Sodium 1g, disodium hydrogen phosphate 1g;
[0051] The preparation method of described ulinastatin freeze-dried powder preparation comprises the following steps:
[0052] (1) Weigh disodium hydrogen phosphate and sodium dihydrogen phosphate in the liquid mixing tank according to the dosage of the formula, stir with water for injection at room temperature until dissolved, and this solution is an auxiliary material solution; dissolve the amount of the above formula with water for injection at room temperature Ulinastatin, and mixed with the above-mentioned adjuvant solution, then add mannitol, sodium chloride, to obtain the medicinal solution A, all the medicinal solutions involved in the following steps (2)-(5) are kept at room temperature;
[00...
Embodiment 3
[0063] A preparation method of ulinastatin freeze-dried powder preparation, the formula of ulinastatin freeze-dried powder preparation is, per 1000: ulinastatin 100 million units, mannitol 20g, sodium chloride 2g, dihydrogen phosphate Sodium 2g, disodium hydrogen phosphate 2g; The preparation method of described ulinastatin freeze-dried powder preparation comprises the following steps:
[0064] (1) Weigh disodium hydrogen phosphate and sodium dihydrogen phosphate in the liquid mixing tank according to the dosage of the formula, stir with water for injection at room temperature until dissolved, and this solution is an auxiliary material solution; dissolve the amount of the above formula with water for injection at room temperature Ulinastatin, and mixed with the above-mentioned adjuvant solution, then add mannitol, sodium chloride, to obtain the medicinal solution A, all the medicinal solutions involved in the following steps (2)-(5) are kept at room temperature;
[0065] (2) R...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com