Ulinastatin purification method based on affinity chromatography column
A technology of ulinastatin and a purification method, which is applied in the field of ulinastatin purification based on an affinity chromatography column, can solve problems such as high technical content, achieve simple operation, reduce production cost, and improve production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
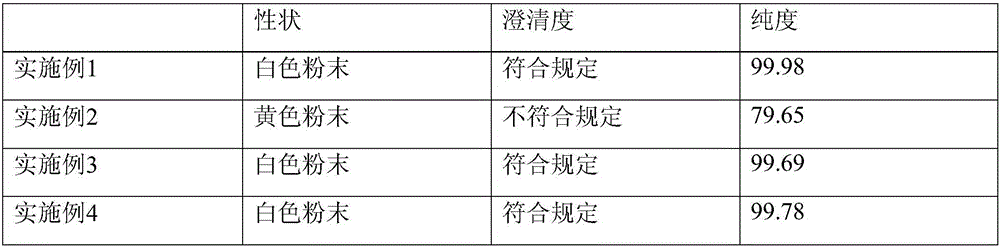
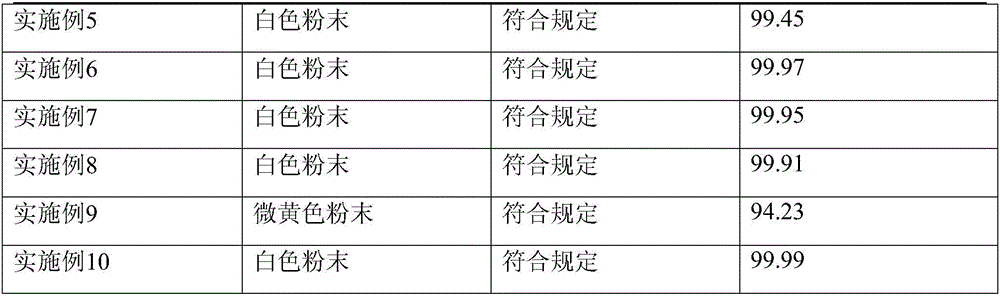
Embodiment 1
[0050] (1) Weigh 1TpH less than 6.5 clarified urine, stir continuously, slowly add 10kg chitosan, and measure with precision pH test paper, use acetic acid to adjust the urine pH to 6.0; 3.5kg of ammonium sulfate was salted out, left standing for 24 hours, placed in a vacuum desiccator with anhydrous magnesium sulfate water-absorbing agent after precipitation and centrifugation, and vacuum-dried to obtain the crude product of ulinastatin after drying;
[0051] (2) Hydrophobic column chromatography (XK50 / 20 chromatography column, PhenylSepharose6FastFlow (highsub) filler)
[0052] Pretreatment: Weigh 1kg of crude ulinastatin, dissolve it with 10mmol / LHAc-NaAc, stir for 30 minutes, centrifuge for 20 minutes, keep the centrifugate, adjust pH to 6.0 with acetic acid, add solid ammonium sulfate to adjust cd to 60ms / cm ;
[0053] Balance: use balance buffer (10mmol / LHAc-NaAc buffer, pH6.0, add solid sodium chloride to adjust cd60ms / cm), adjust the pump flow rate to 20.0ml / min, and ...
Embodiment 2
[0068] (1) Weigh 1TpH less than 6.5 clarified urine, stir continuously, slowly add 10kg chitosan, and measure with precision pH test paper, use acetic acid to adjust the urine pH to 6.0; 3.5kg of ammonium sulfate was salted out, left standing for 24 hours, placed in a vacuum desiccator with anhydrous magnesium sulfate water-absorbing agent after precipitation and centrifugation, and vacuum-dried to obtain the crude product of ulinastatin after drying;
[0069] (2) Hydrophobic column chromatography (XK50 / 20 chromatography column, PhenylSepharose6FastFlow (highsub) filler)
[0070] Pretreatment: Weigh 1kg of crude ulinastatin, dissolve it with 10mmol / LHAc-NaAc, stir for 30 minutes, centrifuge for 20 minutes, keep the centrifugate, adjust pH to 6.0 with acetic acid, add solid ammonium sulfate to adjust cd to 60ms / cm ;
[0071] Balance: use balance buffer (10mmol / LHAc-NaAc buffer, pH6.0, add solid sodium chloride to adjust cd60ms / cm), adjust the pump flow rate to 20.0ml / min, and ...
Embodiment 3
[0082] (1) Weigh 1TpH less than 6.5 clarified urine, stir continuously, slowly add 10kg chitosan, and measure with precision pH test paper, use acetic acid to adjust the urine pH to 6.0; 3.5kg of ammonium sulfate was salted out, left standing for 24 hours, placed in a vacuum desiccator with anhydrous magnesium sulfate water-absorbing agent after precipitation and centrifugation, and vacuum-dried to obtain the crude product of ulinastatin after drying;
[0083] (2) Hydrophobic column chromatography (XK50 / 20 chromatography column, PhenylSepharose6FastFlow (highsub) filler)
[0084] Pretreatment: Weigh 1kg of crude ulinastatin, dissolve it with 10mmol / LHAc-NaAc, stir for 30 minutes, centrifuge for 20 minutes, keep the centrifugate, adjust pH to 6.0 with acetic acid, add solid ammonium sulfate to adjust cd to 60ms / cm ;
[0085] Balance: use balance buffer (10mmol / LHAc-NaAc buffer, pH6.0, add solid ammonium sulfate to adjust cd60ms / cm), adjust the pump flow rate to 20.0ml / min, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com