Use of ulinastatin in preparation of medicament for treating systemic lupus erythematosus and medicinal composition of ulinastatin
A technology for ulinastatin and lupus erythematosus, which is used in the drug for the treatment of systemic lupus erythematosus. unbearable problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Embodiment 1 prepares ulinastatin dry powder injection
[0013] Take 100 million units of filtered sterilized ulinastatin aqueous solution, add 20 grams of mannitol to dissolve, adjust the pH to neutral, add water for injection to 2000 ml, add sodium chloride to adjust isotonicity, filter aseptically, and pack in 1000 pieces In a vial, freeze-dried under sterile conditions, to obtain.
Embodiment 2
[0014] Embodiment 2 prepares ulinastatin injection
[0015] Take 100 million units of ulinastatin aqueous solution sterilized by filtration, adjust the pH to neutral, add water for injection to 2000 ml, add sodium chloride to adjust isotonicity, filter aseptically, pack in 1000 vials, and obtain.
Embodiment 3
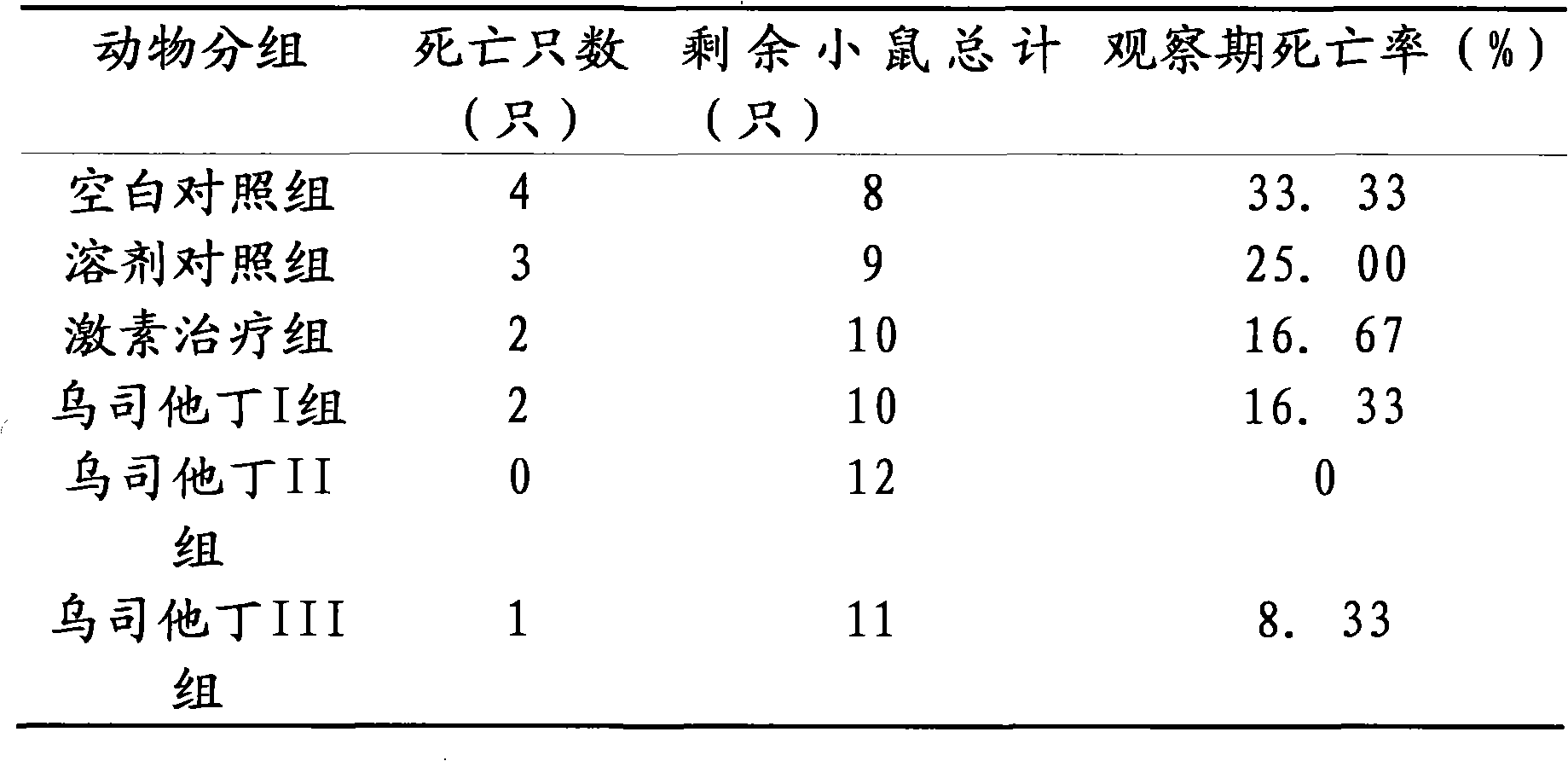
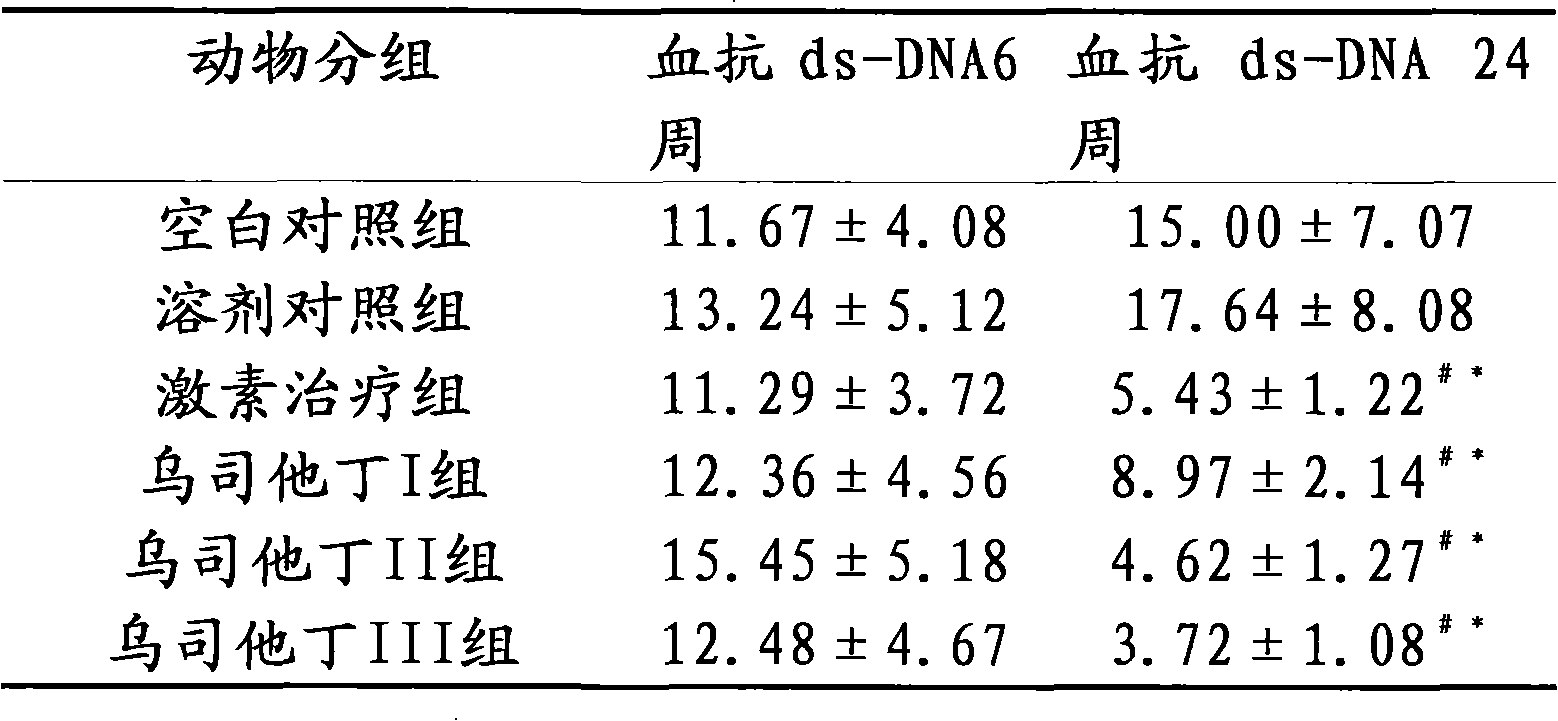
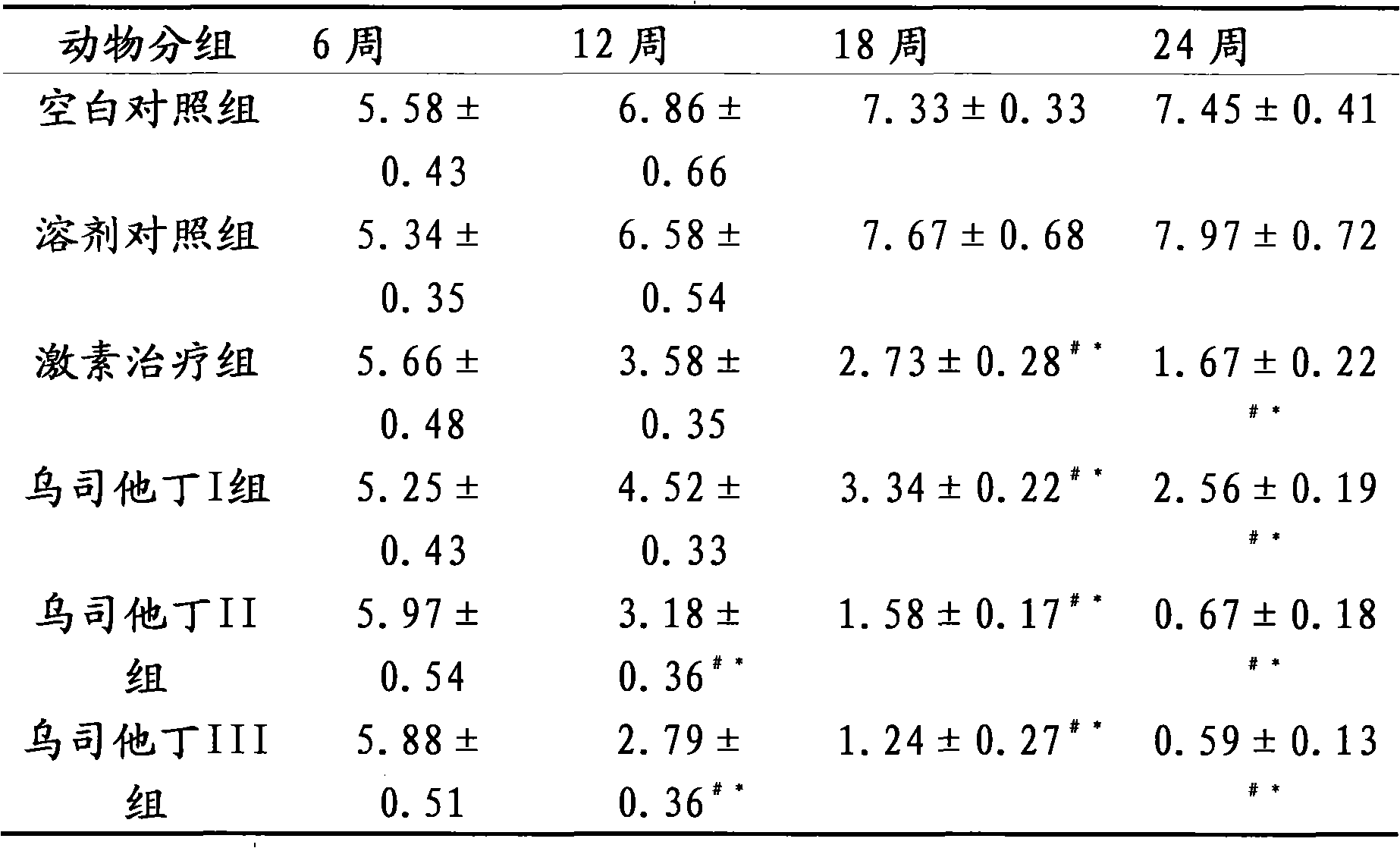
[0016] The therapeutic effect of embodiment 3 ulinastatin on lupus erythematosus mice
[0017] Part 1: Selection and establishment of lupus-like mouse model of chronic graft-versus-host disease
[0018] The mouse model of chronic graft-versus-host disease lupus-like (cGVHD) is a good example of lupus murine nephritis. Several autoantibodies can appear in the model body, including anti-dsDNA, ssDNA, and histone antibodies, and fatal lupus-like nephritis can also appear. The experimental animals are 6-8 weeks old female (C57BL / 6J×DBA / 2) F1 hybrid mice and 6-8 weeks old female DBA / 2 mice, weighing (18-20) g, clean grade. Observation, testing, and evaluation of blood, urine, and basic status were carried out regularly before and after modeling. Under sterile conditions, the spleen, lymph nodes (mesentery, groin, and neck) and thymus of DBA / 2 mice were taken out, cut into pieces, ground, washed with PBS and sieved, and the cell suspension was sieved at a ratio of 1:1. Slowly add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com