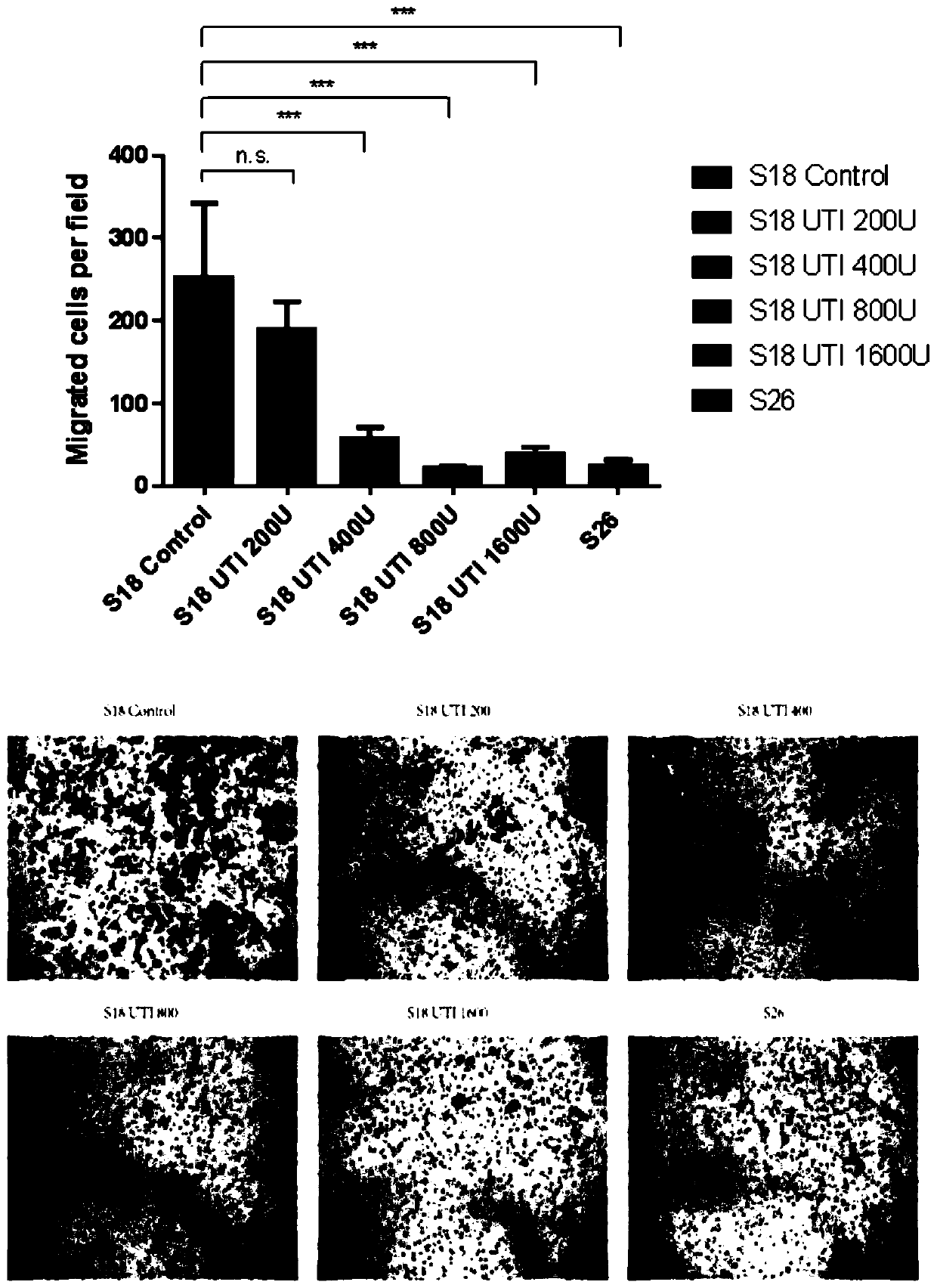
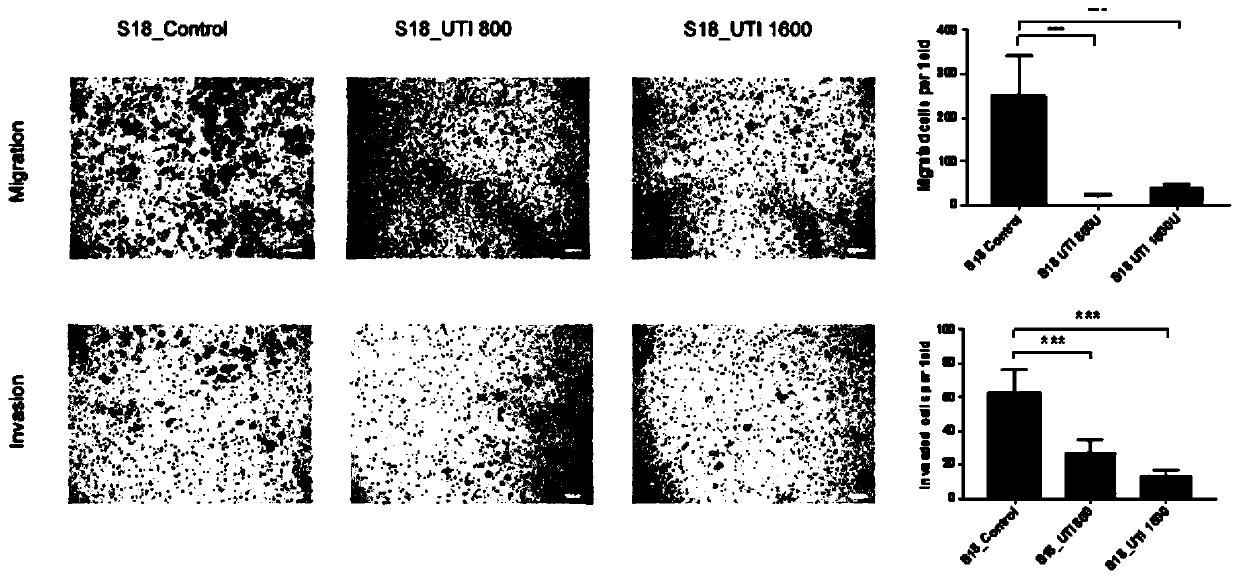
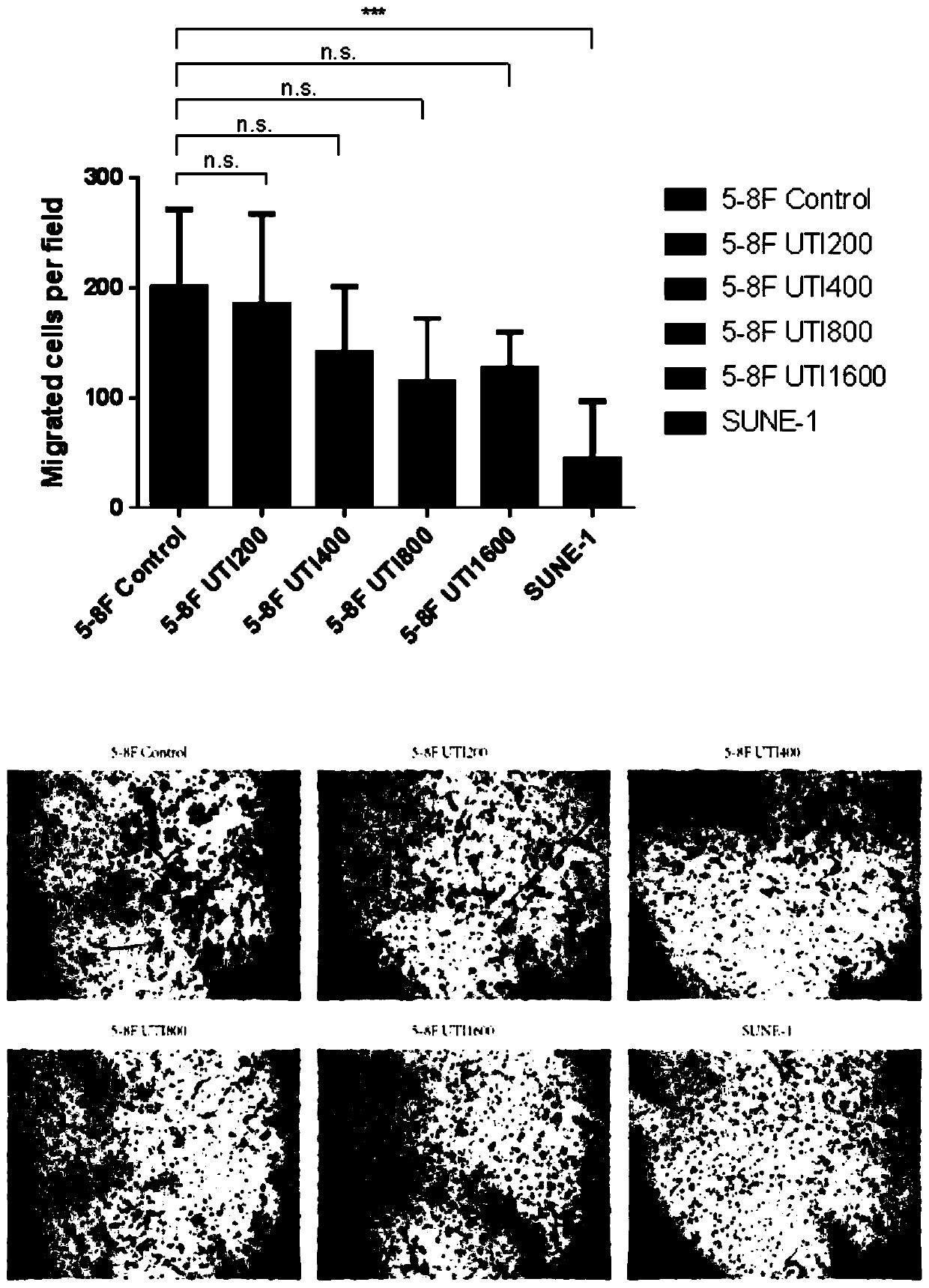
Application of ulinastatin in preparations of anti-nasopharyngeal carcinoma metastasis medicines
A technology for ulinastatin and nasopharyngeal carcinoma, which is applied in the field of medicine to achieve the effects of strong inhibitory effect, inhibition of migration and invasion, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1, a kind of anti-nasopharyngeal carcinoma metastasis drug preparation
[0031] Take filtered sterilized ulinastatin 1000U and mannitol 10g, add it to an appropriate amount of water for injection to dissolve, adjust the pH to 6.8, add water for injection to 1000ml, add sodium chloride to adjust isotonicity, filter aseptically, and dispense Freeze-dried under aseptic conditions in 1000 vials to obtain the product.
Embodiment 2
[0032] Embodiment 2, a kind of anti-nasopharyngeal carcinoma metastasis drug preparation
[0033] Take ulinastatin 2000U, 7g of stachydrine hydrochloride, 2.8g of dextran, and 10g of mannitol after filtration sterilization and add them to an appropriate amount of water for injection to dissolve, adjust the pH to 7.1, add water for injection to 1000ml, add chlorine Sodium chloride is used to adjust isotonicity, filtered aseptically, divided into 1000 vials, and freeze-dried under sterile conditions to obtain the product.
Embodiment 3
[0034] Embodiment 3, a kind of anti-nasopharyngeal carcinoma metastasis drug preparation
[0035] Take ulinastatin 5000U, 17.5g of stachydrine hydrochloride, 7g of dextran, and 5g of mannitol after filter sterilization and add them to an appropriate amount of water for injection to dissolve, adjust the pH to 6.9, add water for injection to 1000ml, add chlorine Sodium chloride is used to adjust isotonicity, filtered aseptically, and divided into 1000 vials to obtain the product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com