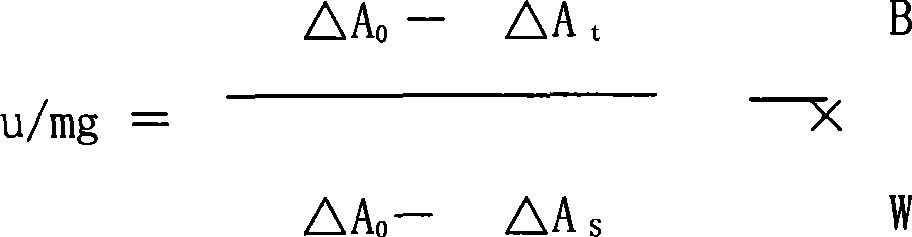Stable water injection medicament composition containing Ulinastatin
A technology of ulinastatin and composition, which is applied in the field of ulinastatin and a certain proportion of excipients, which can solve problems such as microbial contamination, affecting the treatment effect of patients, and reducing biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Preparation of Ulinastatin Injection
[0023] The ulinastatin of the present invention can be prepared according to the preparation method disclosed in the patent "purified ulinastatin and its preparation method and pharmaceutical composition containing the ulinastatin" (ZL200610000200.2). Using fresh human urine as raw material, it is prepared through the following process steps:
[0024] a. Pump the urine into the stirring tank, adjust the pH to 4.5-6, add chitin for adsorption, elution with ammonium sulfate solution, suction filtration of the eluate, and the drained product is a crude Ulinastatin product;
[0025] b. After dissolving the crude Ulinastatin product with 2-3 times of water, filter, take the supernatant, adjust the pH to 6-7.5 and then precipitate with ethanol, dissolve the precipitate with water and filter, and apply the filtrate to an anion exchange column Elute with buffer containing 0.1-2mol / L NaCl and 0.1-0.5mol / L phosphate, and collect the el...
Embodiment 2
[0041] Example 2: Preparation of Ulinastatin Injection
[0042] The prescription 2-10 ratio is as follows:
[0043]
[0044] Prescription 2-10 Ulinastatin injection preparation method: according to the method described in Example 1.
Embodiment 3
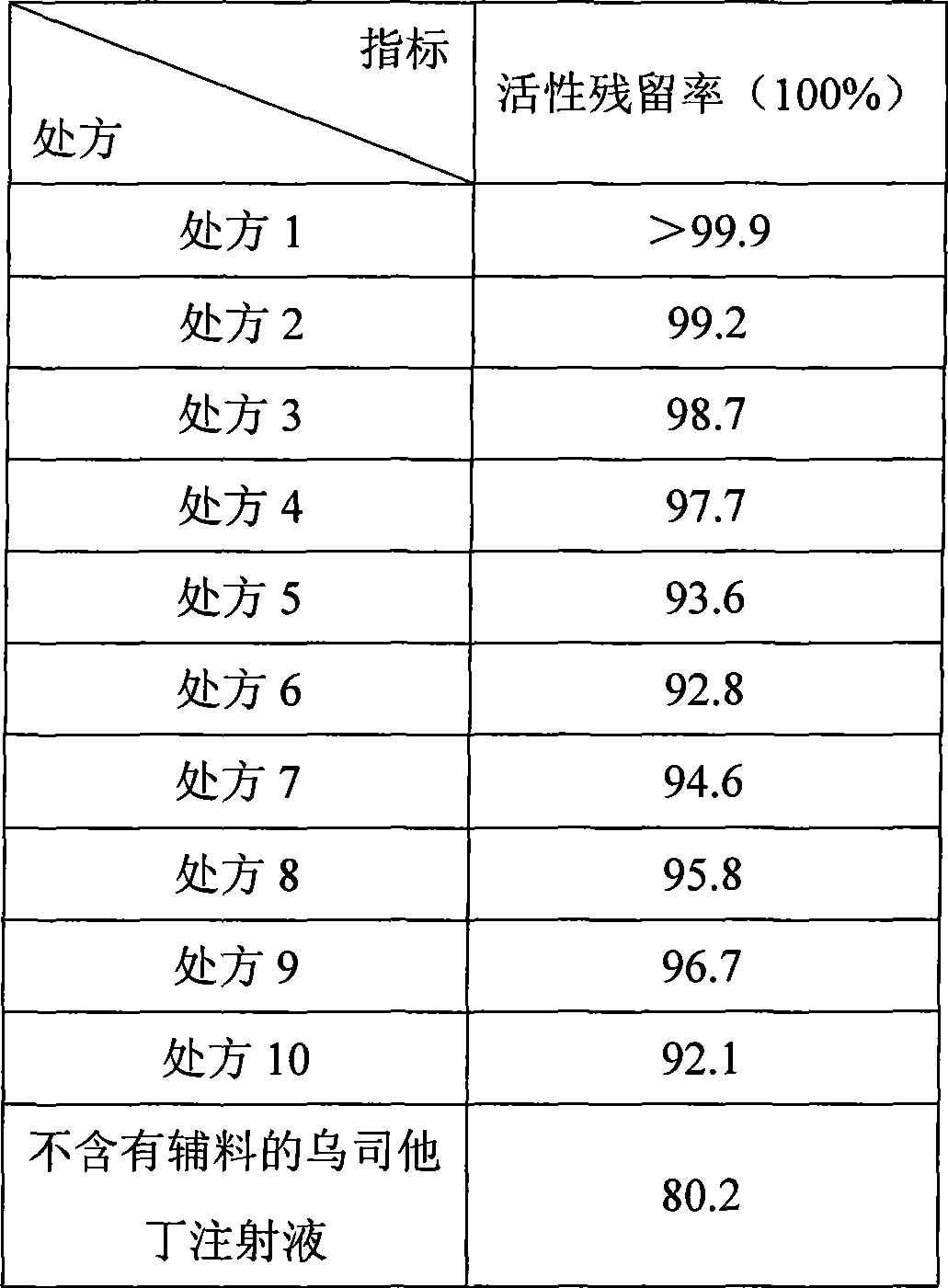
[0045] Example 3 Stability test
[0046] Take one 100,000-unit ulinastatin injection prepared according to the prescription 1-10 method, and one 100,000-unit ulinastatin injection prepared according to the preparation method of Example 1 without excipients ; After being placed at 25°C for 2 years, use the following method to determine the activity of Ulinastatin, and calculate the residual activity of Ulinastatin according to the following formula:
[0047] Residual activity rate = activity after storage / activity before storage×100%
[0048] Ulinastatin activity determination method:
[0049] Preparation of standard solution Weigh an appropriate amount of ulinastatin standard, add 0.2mol / L triethanolamine buffer (take 29.8g of triethanolamine, add 900ml of water to dissolve, adjust pH to 7.8 with 4mol / L hydrochloric acid solution, add water to 1000ml , Shake well, and get it.) Make a solution containing 50 units per 1ml.
[0050] Preparation of the test solution: Weigh an appropri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com