Ulinastatin purification method based on hydrophobic column
A technology of ulinastatin and hydrophobic column, which is applied in the biological field, can solve the problems of low adsorption efficiency, long production cycle, and low product yield of the purification method, and achieve simple and feasible purification process, low economic cost, and improved purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
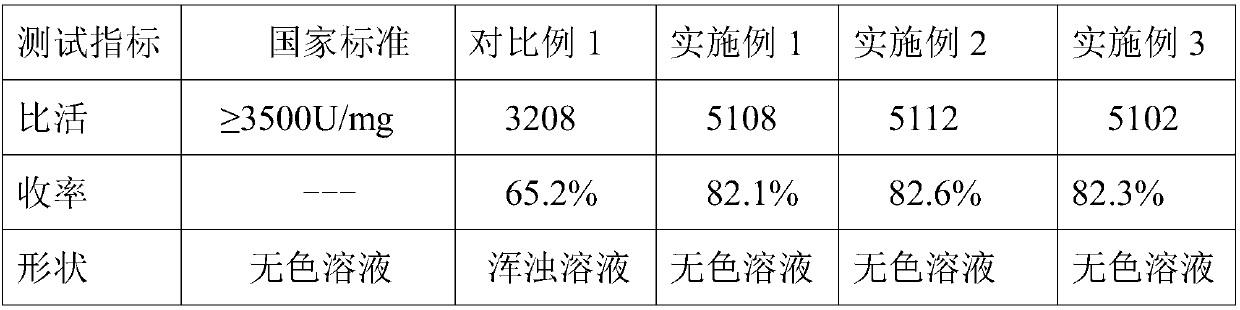
Embodiment 1
[0020] A method for purifying ulinastatin based on a hydrophobic column, the specific steps are as follows:
[0021] S1: Dissolve the crude product of ulinastatin together with purified water in a beaker, then add an appropriate amount of salts into the beaker, and control the pH of the solution in the beaker to be 5, put it on a magnetic stirrer and stir evenly for 60min, and use Filter through a strainer to obtain sediment and set aside.
[0022] S2: Clean and dry the hydrophobic column, fix it on the operating table, take the precipitate prepared in step S1, dissolve it in 1 times deionized water and mix evenly, add it to the upper end of the hydrophobic column and react for 1 hour, then , add 1mol / L salt buffer for washing.
[0023] S3: Next, wash and filter with a low-concentration buffer solution to obtain a sample of ulinastatin, and finally, put the sample of ulinastatin into a freezer for freezing until freeze-drying.
[0024] S4: Wash the hydrophobic column used in...
Embodiment 2
[0032] A method for purifying ulinastatin based on a hydrophobic column, the specific steps are as follows:
[0033] S1: Dissolve the crude product of ulinastatin and purified water together in a beaker, then add an appropriate amount of salts to the beaker, and control the pH of the solution in the beaker to be 5.5, put it on a magnetic stirrer and stir evenly for 100min, and use Filter through a strainer to obtain sediment and set aside.
[0034] S2: Clean and dry the hydrophobic column, fix it on the operating table, take the precipitate prepared in step S1, dissolve it in 1.5 times deionized water and mix evenly, then add it evenly to the upper end of the hydrophobic column and react for 1.5h, Next, add 1.5mol / L salt buffer solution for washing.
[0035] S3: Next, wash and filter with a low-concentration buffer solution to obtain a sample of ulinastatin, and finally, put the sample of ulinastatin into a freezer for freezing until freeze-drying.
[0036] S4: Wash the hydr...
Embodiment 3
[0044] A method for purifying ulinastatin based on a hydrophobic column, the specific steps are as follows:
[0045] S1: Dissolve the crude product of ulinastatin together with purified water in a beaker, then add an appropriate amount of salt in the beaker, and control the pH of the solution in the beaker to be 6, put it on a magnetic stirrer and stir evenly for 150min, and use Filter through a strainer to obtain sediment and set aside.
[0046] S2: Clean and dry the hydrophobic column, fix it on the operating table, take the precipitate prepared in step S1, dissolve it in 2 times deionized water and mix evenly, then add it evenly to the upper end of the hydrophobic column and react for 2 hours, then , add 2mol / L salt buffer for washing.
[0047] S3: Next, wash and filter with a low-concentration buffer solution to obtain a sample of ulinastatin, and finally, put the sample of ulinastatin into a freezer for freezing until freeze-drying.
[0048] S4: Wash the hydrophobic col...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com