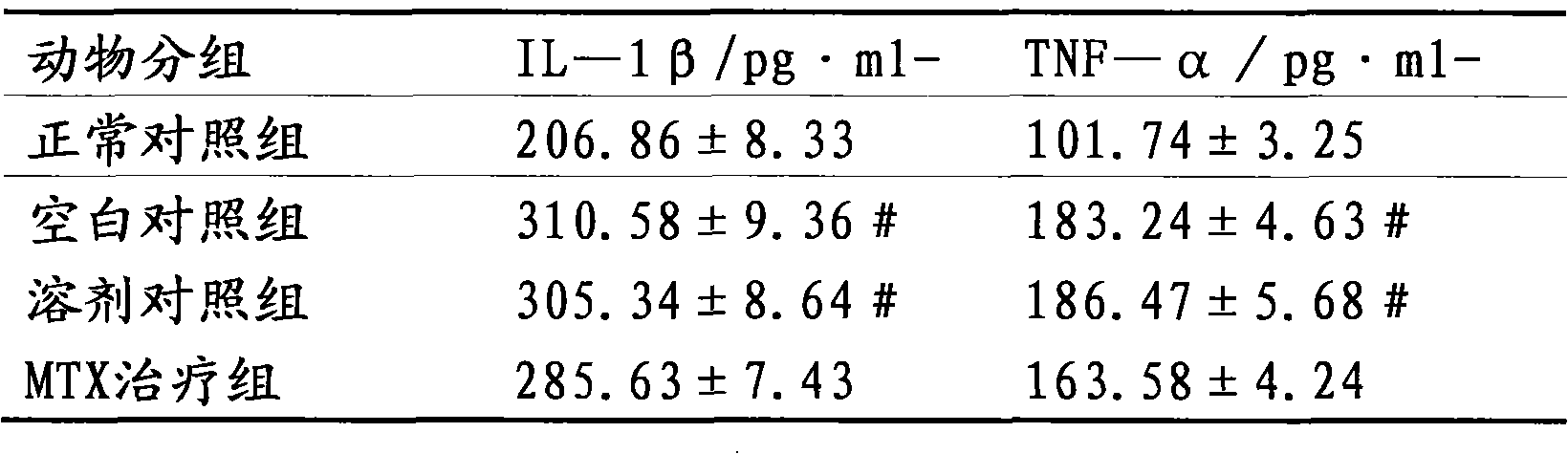Use of ulinastatin in preparation of drugs for treating rheumatoid arthritis and pharmaceutical composition thereof
A ulinastatin and rheumatoid technology, applied in the field of medicinal chemistry, can solve the problems of severe clinical adverse reactions, failure to prevent disease progression, and no specific and effective treatment methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0011] Embodiment 1 prepares ulinastatin dry powder injection
[0012] Take 100 million units of filtered sterilized ulinastatin aqueous solution, add 20 grams of mannitol to dissolve, adjust the pH to neutral, add water for injection to 2000 ml, add sodium chloride to adjust isotonicity, filter aseptically, and pack in 1000 pieces In a vial, freeze-dried under sterile conditions, to obtain.
Embodiment 2
[0013] Embodiment 2 prepares ulinastatin injection
[0014] Take 100 million units of ulinastatin aqueous solution sterilized by filtration, adjust the pH to neutral, add water for injection to 2000 ml, add sodium chloride to adjust isotonicity, filter aseptically, pack in 1000 vials, and obtain.
Embodiment 3
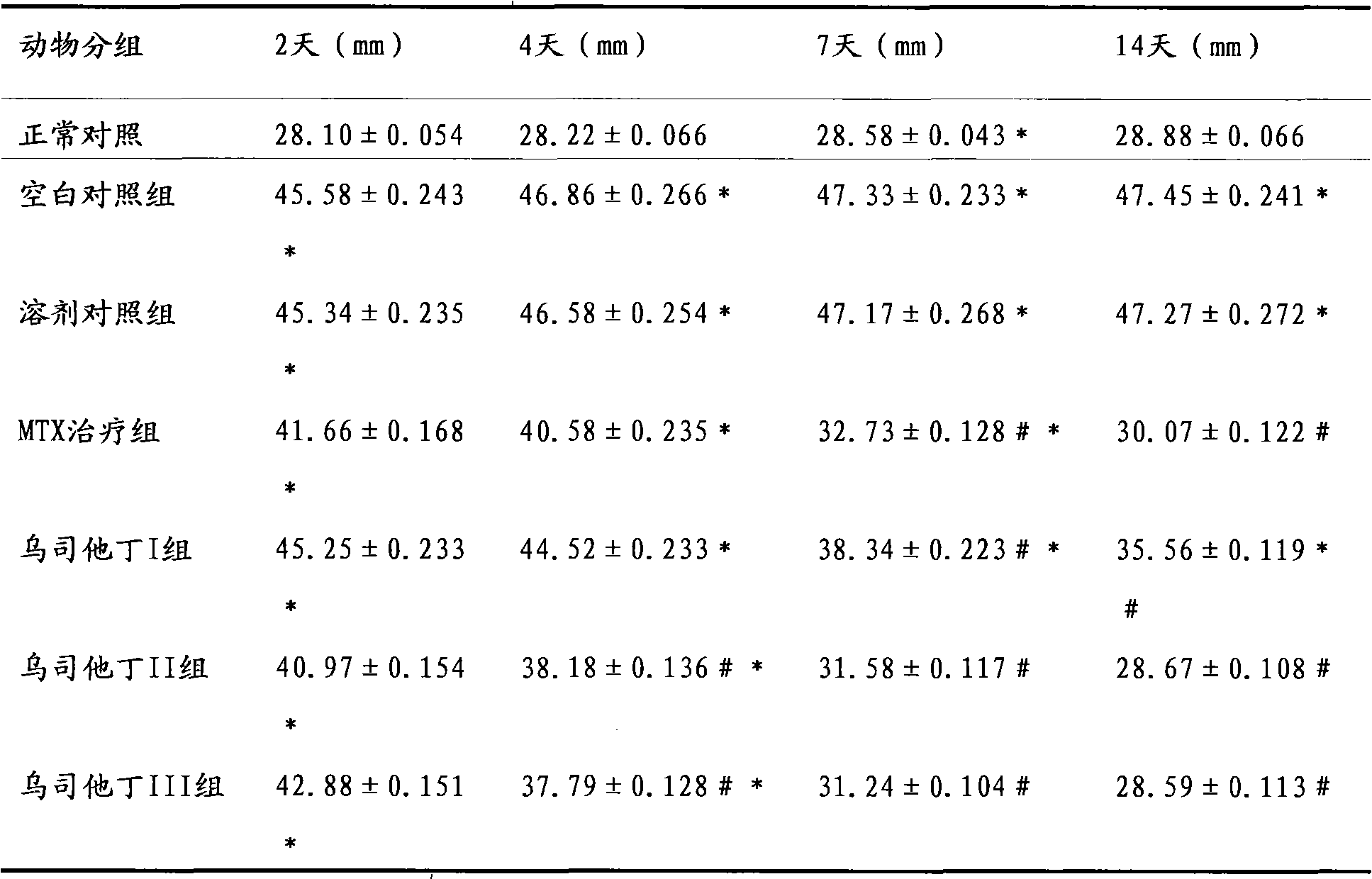
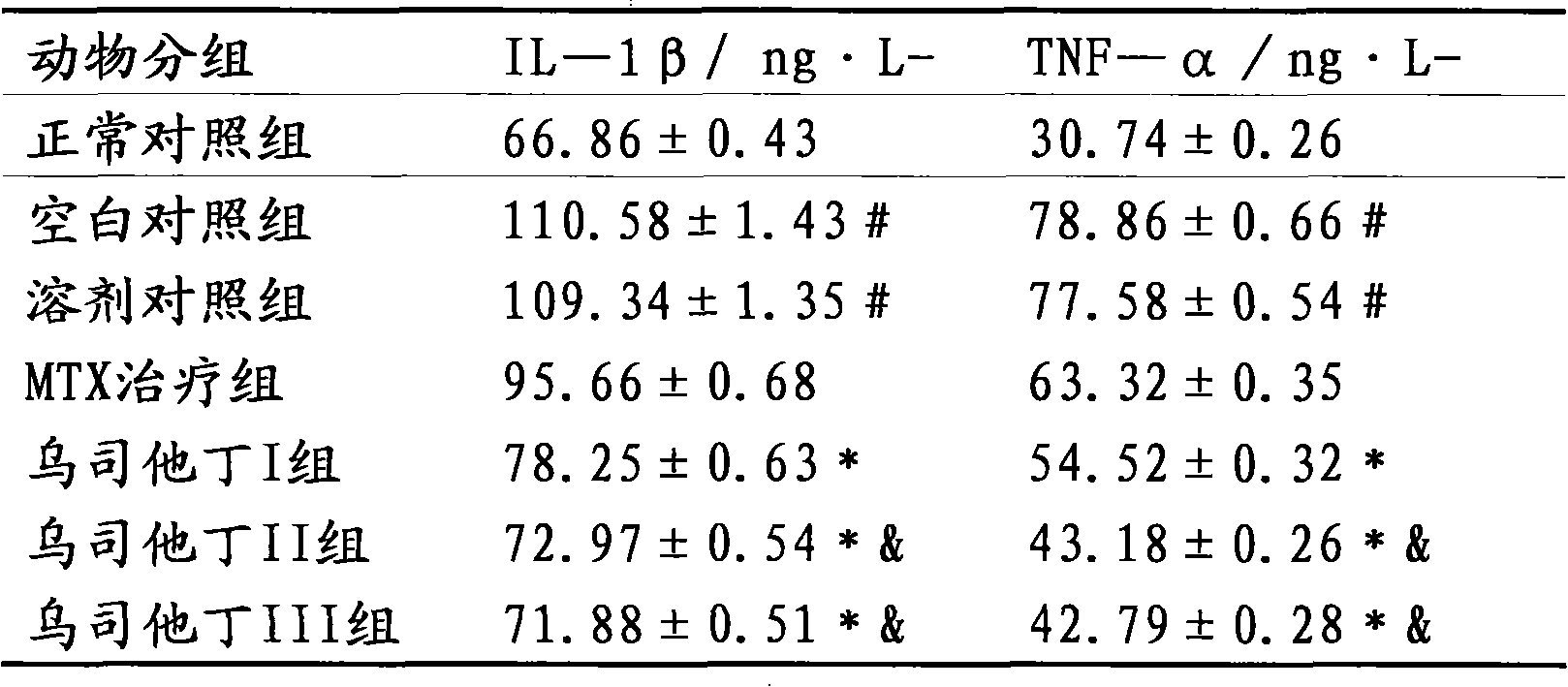
[0015] The therapeutic effect of embodiment 3 ulinastatin on rheumatoid arthritis rats
[0016] Part 1: Establishment and Scoring of Collagen-Induced Arthritis (CIA) Animal Model
[0017] Dissolve bovine type II collagen (C II) in 0.1M acetic acid to make a 2mg / ml solution, mix it with an equal volume of complete Freund's adjuvant, stir and emulsify with magnetic beads, and prepare an emulsion with a final concentration of 1mg / ml. Take 0.5ml (containing C II 0.5mg) and inject intradermally at 5 parts of the back of Wistar rats, and 10 days later intradermally inject the same dose at the base of the tail of the rats to boost the immunization once. Observe the incidence of arthritis in the rats, and score according to the degree of redness and swelling of the legs and toes of the arthritis-onset rats (grade 0-4). 0 points: no arthritis; 1 point: red spots or mild swelling; 2 points: moderate joint swelling; 3 points: severe joint swelling; 4 points: severe redness, severe dysfu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com