Use of ulinastatin in preparation of medicament for treating systemic lupus erythematosus and medicinal composition of ulinastatin
A technology of ulinastatin and lupus erythematosus, which is applied to drugs for the treatment of systemic lupus erythematosus. Ulinastatin, as an application field of drugs for the treatment of systemic lupus erythematosus, can solve problems such as side effects, infection, and unbearable patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0012] Example 1 Preparation of Ulinastatin dry powder injection
[0013] Take 100 million units of filtered sterilized ulinastatin aqueous solution, add 20 grams of mannitol to dissolve, adjust the pH to neutral, add water for injection to 2000 ml, add sodium chloride to adjust isotonicity, sterile filter, divide into 1000 In a vial, freeze-dried under sterile conditions.
Embodiment 2
[0014] Example 2 Preparation of Ulinastatin Injection
[0015] Take 100 million units of the filtered sterilized ulinastatin aqueous solution, adjust the pH to neutral, add water for injection to 2000 ml, add sodium chloride to adjust isotonicity, sterile filter, and divide into 1000 vials to obtain the final product.
Embodiment 3
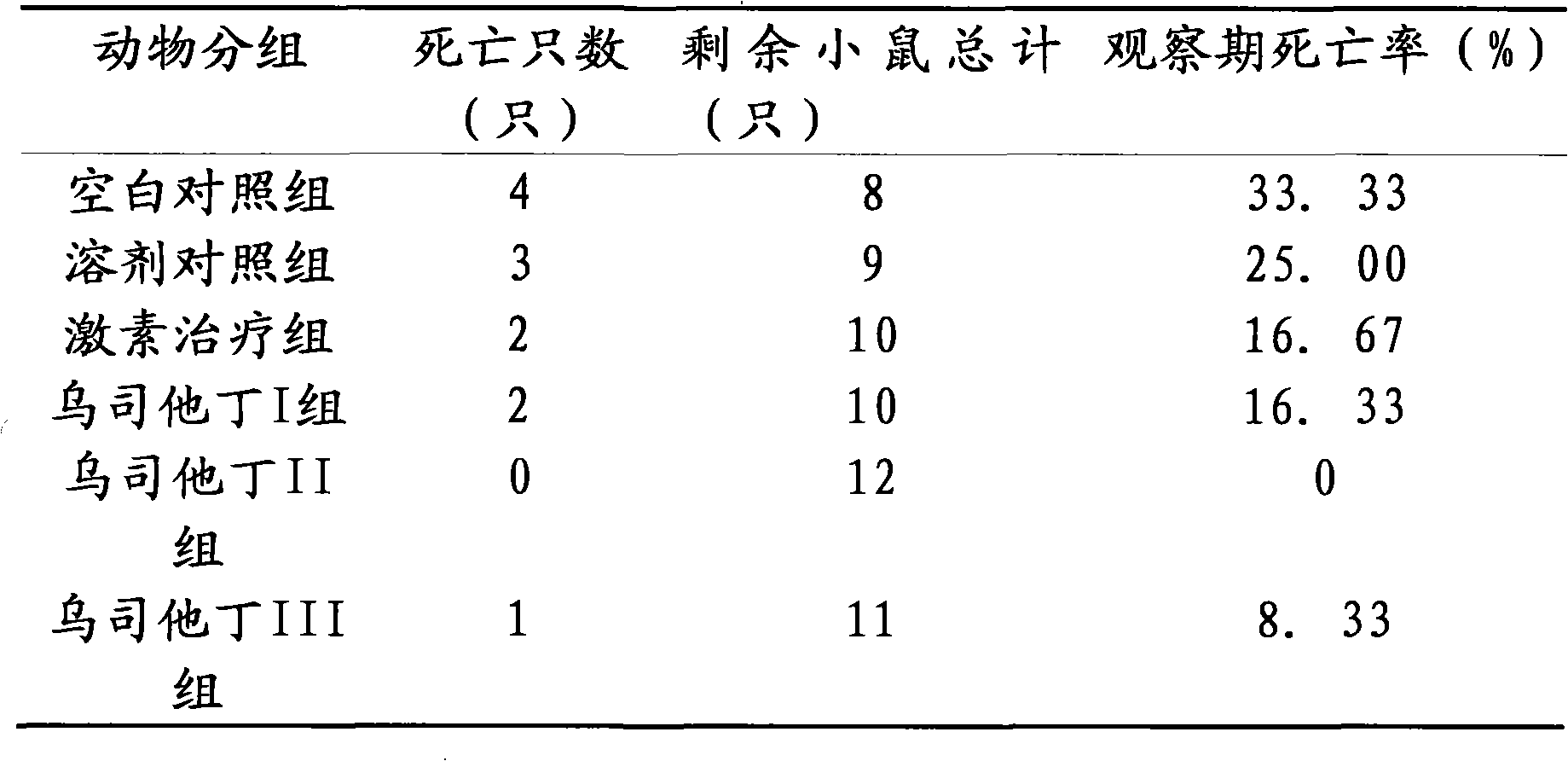
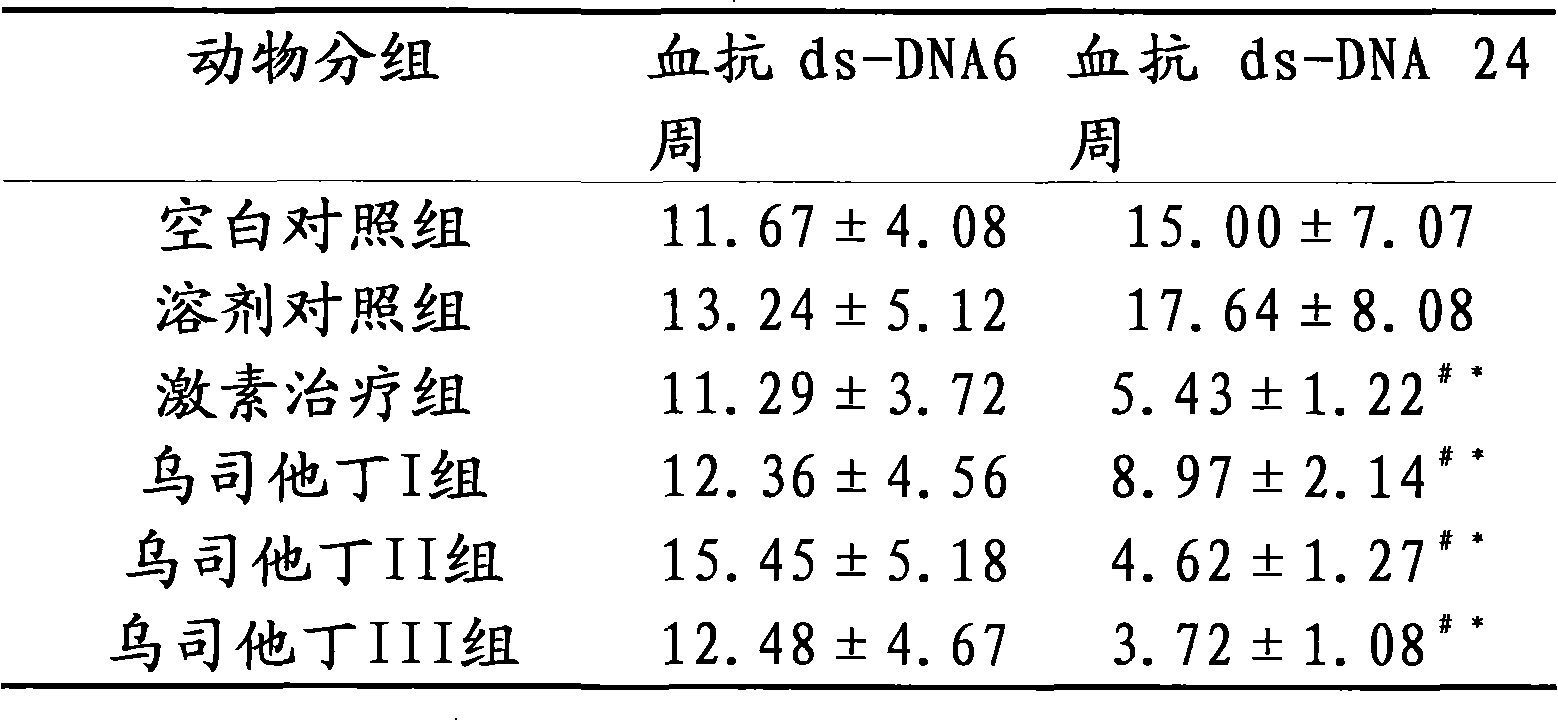
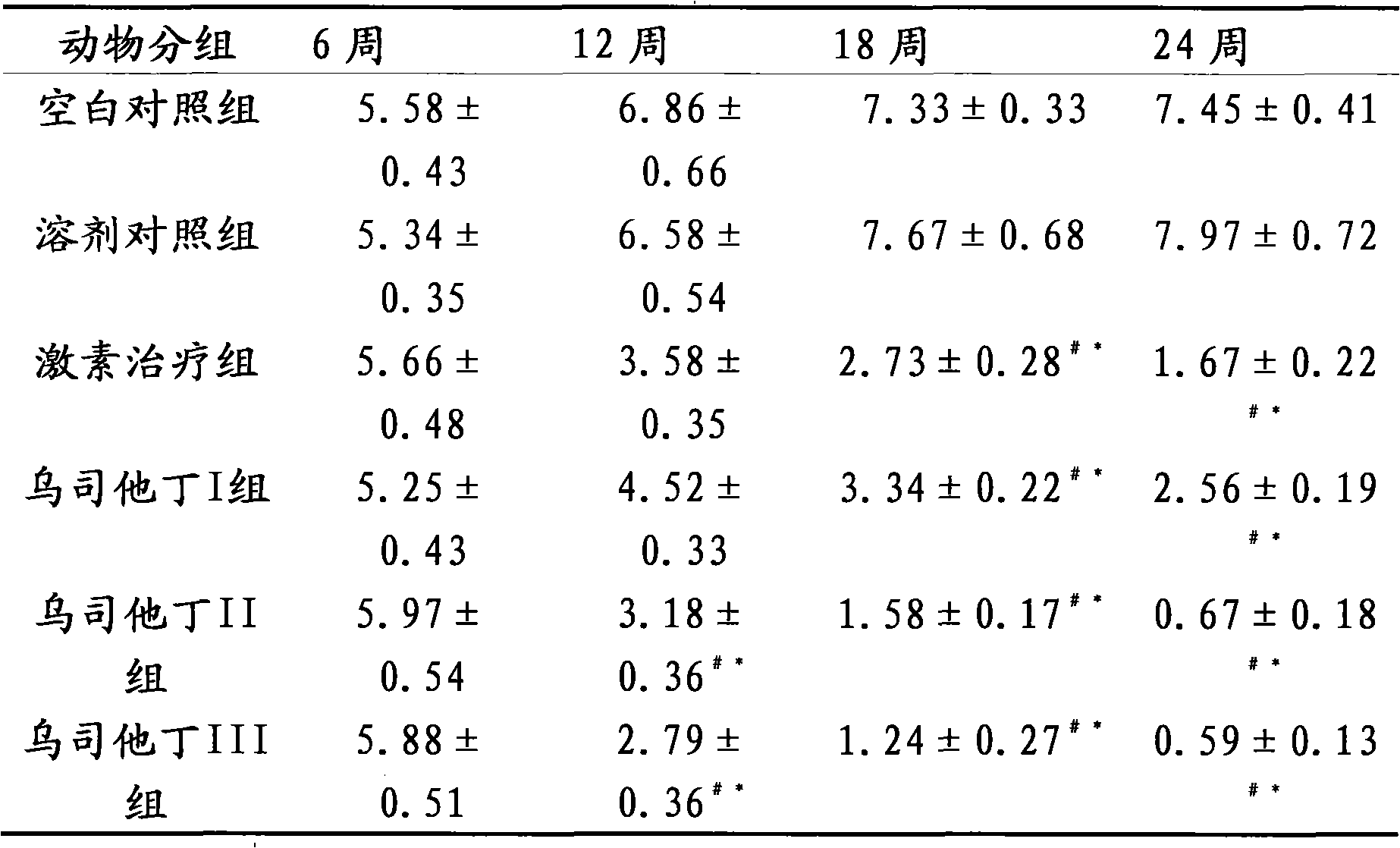
[0016] Embodiment 3 The therapeutic effect of ulinastatin on lupus erythematosus mice
[0017] Part 1: Selection and establishment of a lupus-like mouse model of chronic graft-versus-host disease
[0018] The chronic graft-versus-host disease lupus-like (cGVHD) mouse model is a good example of lupus murine nephritis. Several autoantibodies, including anti-dsDNA, ssDNA, and histone antibodies, can appear in the model, and fatal lupus-like nephritis can also occur. Experimental animals 6-8-week-old female (C57BL / 6J×DBA / 2) F1 hybrid mice and 6-8-week-old female DBA / 2 mice, body weight (18-20) g, clean grade. Before and after modeling, blood, urine and basic status were observed, tested and evaluated regularly. Under sterile conditions, the spleen, lymph nodes (mesenteric, inguinal, and neck) and thymus of DBA / 2 mice were removed, minced, ground, washed with PBS, and sieved. The wall of the centrifuge tube was slowly added on top of the mouse lymphocyte separation solution, cen...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com