Ulinastatin purification method based on hydrophobic interaction column
A technology of ulinastatin and hydrophobic column, which is applied in the field of medicine, can solve the problems of low yield, low purity and instability of ulinastatin, and achieve high yield, huge economic and social benefits, and stability Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
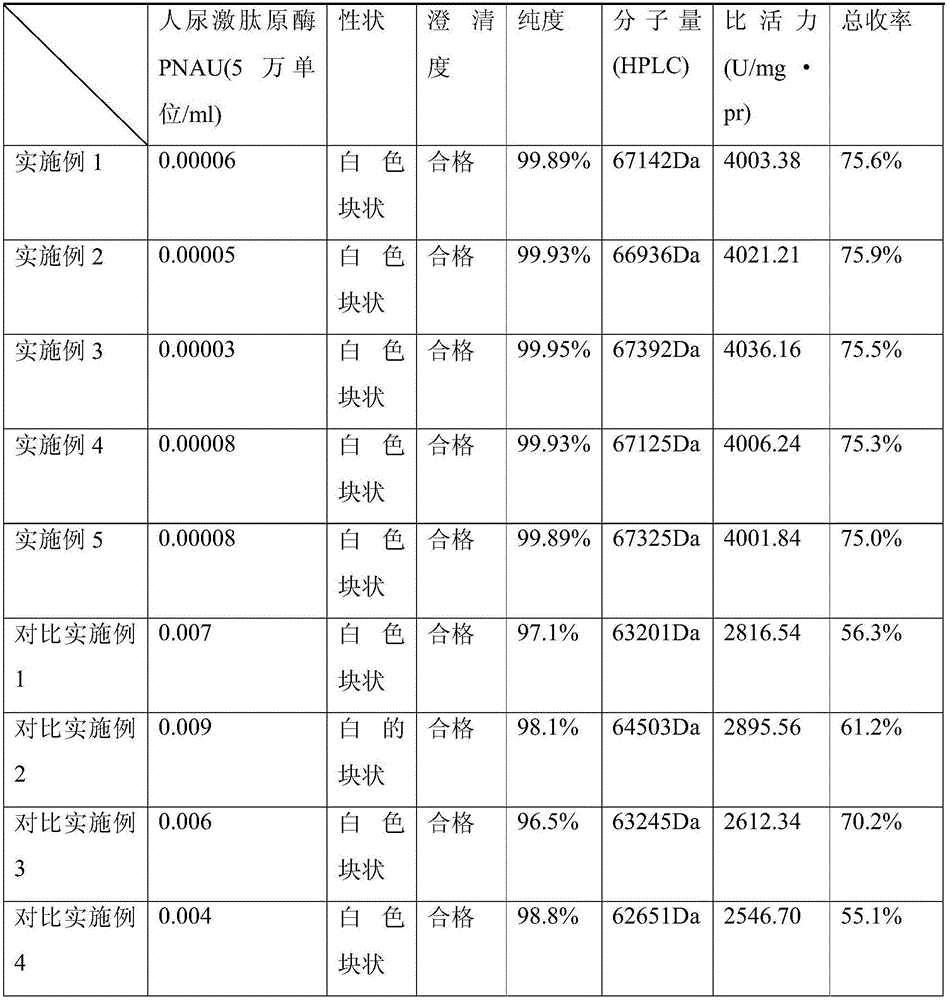
Embodiment 1
[0028] Ulinastatin crude product purification steps:
[0029] 1) Pretreatment: Dissolve 100g of crude ulinastatin in 200ml of water, filter with diatomaceous earth, collect the filtrate, add ZnCl 2 Make its final concentration 150mmol / L, precipitate with precooled 95% ethanol according to the volume ratio of sample solution: 95% ethanol as 1: 1.1 (ml / ml), stir for 30min, plate and frame filter, get the supernatant, Adjust conductance to 55ms / cm, adjust pH to 4.0 with glacial acetic acid. The volume of the sample solution: 95% ethanol is 1:2.3, stirred for 30 minutes, filtered with a plate and frame, and the precipitate is taken; the precipitate is dissolved in 3 times the volume of water.
[0030] 2) Loading and washing: use pH 4.0, 1.5mol / L (NH 4 ) 2 SO 4 - 0.15mol / L acetate buffer equilibrates the chromatography column PhenylBio-sepHP, loads the sample, the protein concentration of the sample solution is about 30mg / mL gel, use pH4.0, 1.5mol / L (NH 4 ) 2 SO 4 - Wash wit...
Embodiment 2
[0033] Ulinastatin crude product purification steps:
[0034] 1) Pretreatment: Dissolve 100g of crude ulinastatin in 200ml of water, filter with diatomaceous earth, collect the filtrate, add ZnCl 2Make its final concentration 150mmol / L, precipitate with precooled 95% ethanol by the volume ratio of sample solution: 95% ethanol as 1: 1.1 (ml / ml), stir for 30min, centrifuge at 5000r / min for 20min, take the supernatant solution, adjust the conductance to 55ms / cm, and adjust the pH to 3.5 with glacial acetic acid. The volume of the sample solution: 95% ethanol is 1:2.3, stirred for 30 minutes, filtered with a plate and frame, and the precipitate is taken; the precipitate is dissolved in 3 times the volume of water.
[0035] 2) Loading and washing: use pH 3.6, 1.5mol / L (NH 4 ) 2 SO 4 - 0.1mol / L acetate buffer equilibrates the ButylBio-sepHP chromatography column, loads the sample, the protein concentration of the sample solution is about 30mg / mL gel, and uses pH3.6, 1.5mol / L (NH...
Embodiment 3
[0038] Ulinastatin crude product purification steps:
[0039] 1) Pretreatment: Dissolve 100g of crude ulinastatin in 200ml of water, filter with diatomaceous earth, collect the filtrate, add ZnCl 2 Make its final concentration 150mmol / L, precipitate with precooled 95% ethanol by the volume ratio of sample solution: 95% ethanol as 1: 1.1 (ml / ml), stir for 30min, centrifuge at 5000r / min for 20min, take the supernatant solution, adjust the conductance to 55ms / cm, and adjust the pH to 4.5 with glacial acetic acid. The volume of the sample solution: 95% ethanol is 1:2.3, stirred for 30 minutes, filtered with a plate and frame, and the precipitate is taken; the precipitate is dissolved in 3 times the volume of water.
[0040] 2) Loading and washing: use pH 3.5, 1.5mol / L (NH 4 ) 2 SO 4 -0.1mol / L acetate buffer equilibrates the chromatography column PhenylBio-sepFF, loads the sample, the protein concentration of the sample solution is about 30mg / mL gel, and uses pH 4.5, 1.5mol / L (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com