Human urinary trypsin inhibitor (hUTI) of reorganization-dimerization and preparation method and application thereof
A protein and amino acid technology, applied in the fields of molecular biology and medicine, can solve the problems of low rhUTI expression, poor activity, and short half-life in vivo
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1. Construction of gene expression vector encoding hUTI-L-vFc recombinant dimerization protein
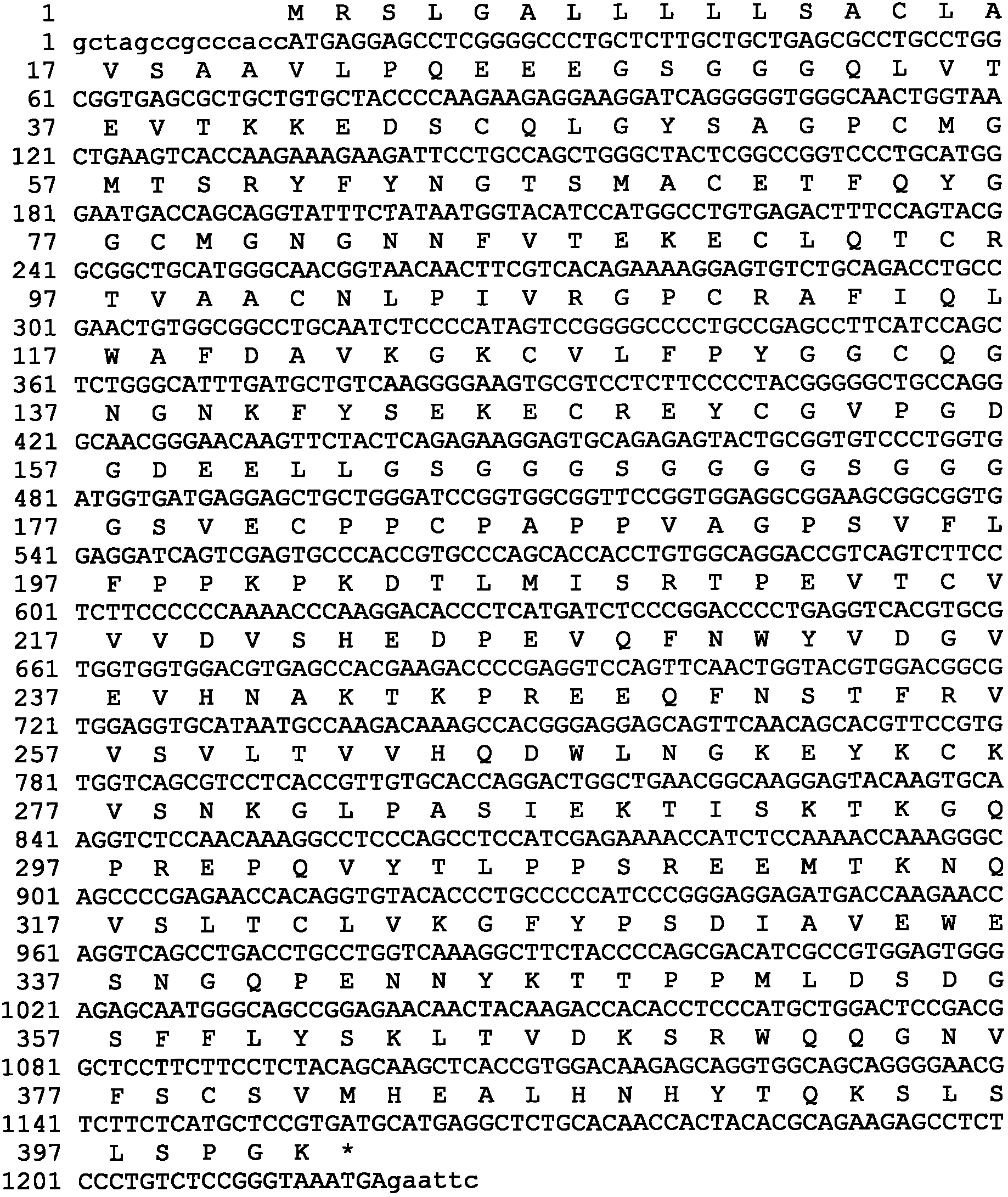
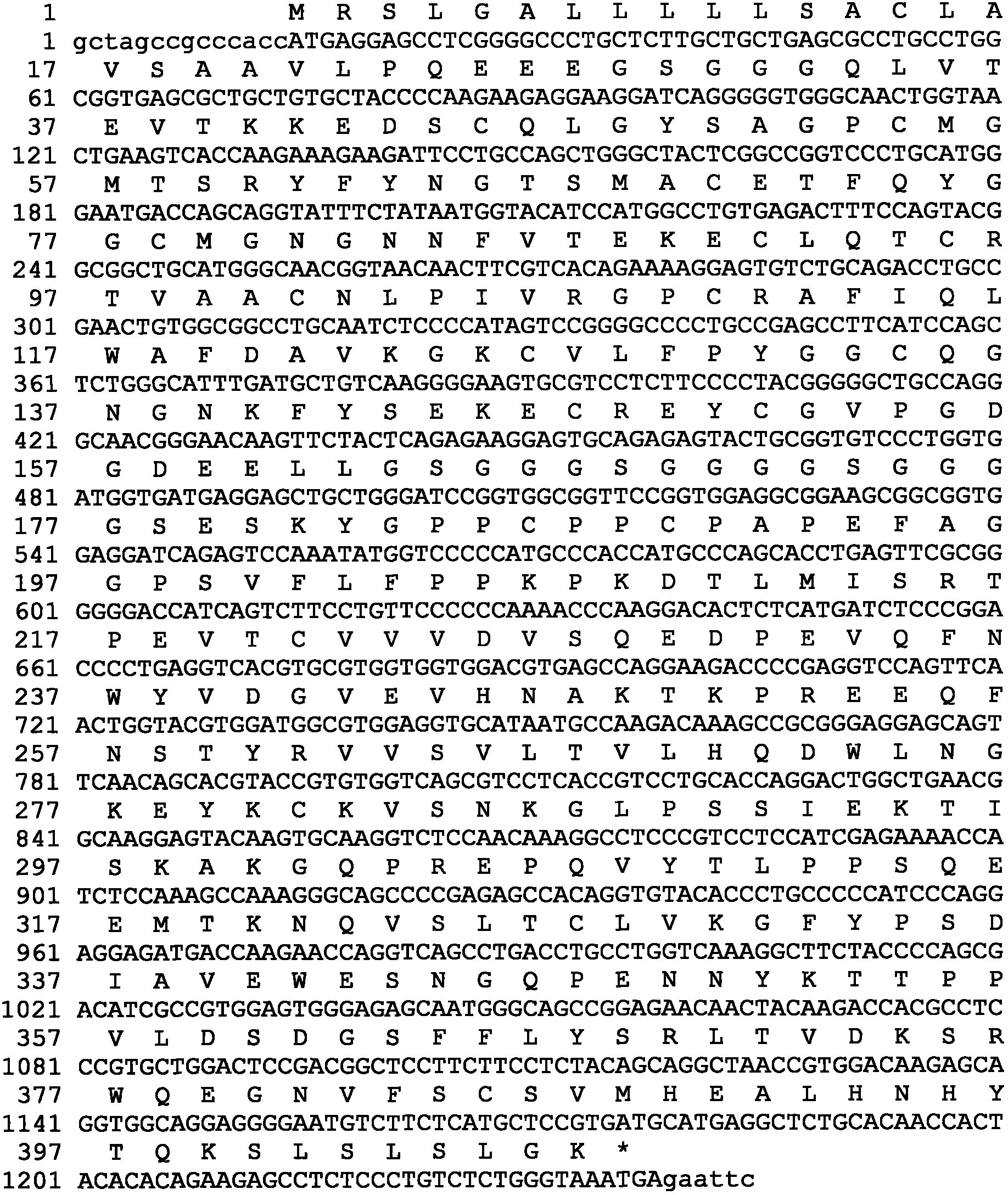
[0082] The gene encoding hUTI leader peptide and mature protein is obtained by artificial synthesis, and the synthesized 506bp DNA fragment is inserted between the EcoRV restriction enzyme sites in the transfer vector such as pUC57 to obtain the phUTI plasmid, which contains the hUTI gene Sequences can be verified by DNA sequencing.
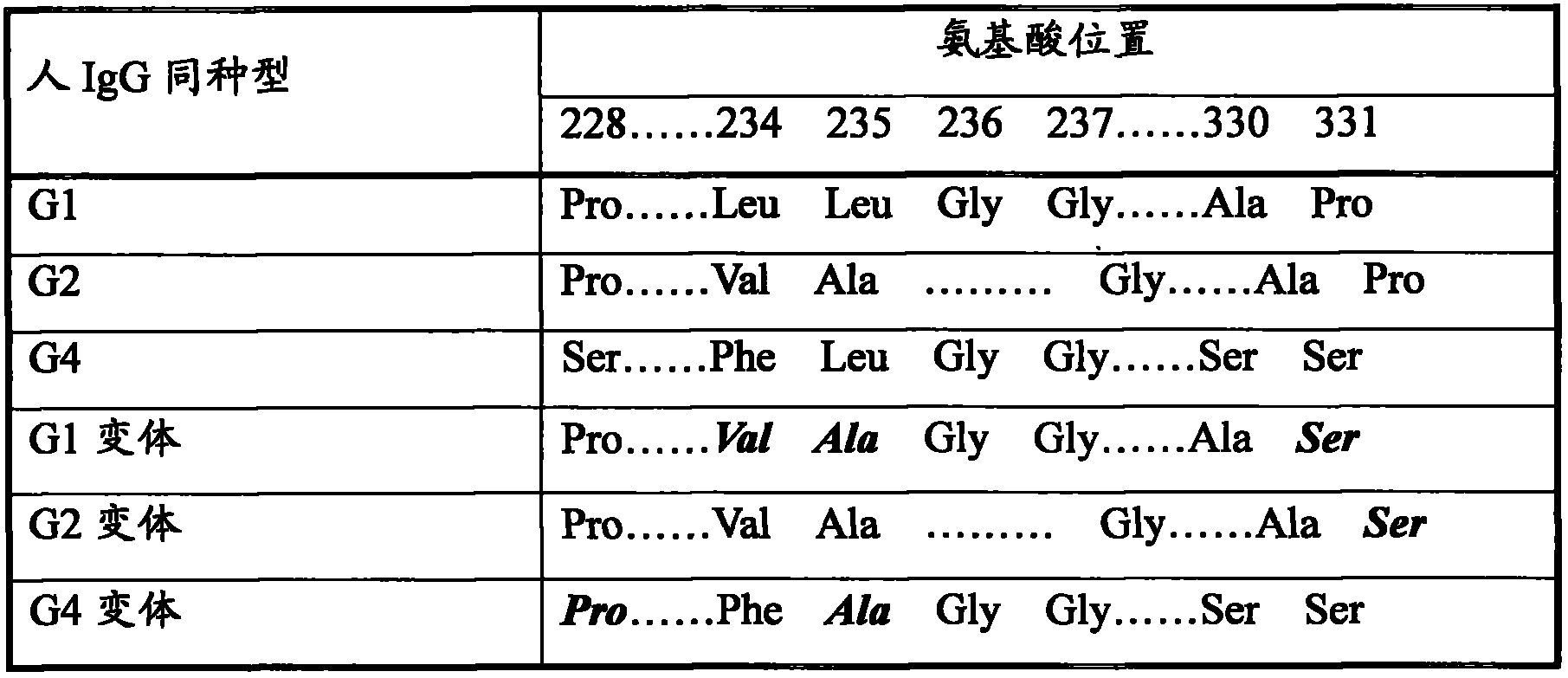
[0083] Flexible Peptide Linker and Human IgG Fc Region Fc γ2 variant vFc γ2 (Pro331Ser mutation), Fc γ4 variant vFc γ4 (Ser228Pro and Leu235Ala mutations), Fc γ1 variant vFc γ1 (Leu234Val, Leu235Ala and Pro331SSer mutations) fusion genes were obtained by artificial synthesis, and the 5' and 3' ends of the synthesized fragments each had a restriction enzyme endonuclease site, respectively BamHI and EcoRI. The obtained DNA fragment is inserted between the BamHI and EcoRI sites of a transfer vector such as PUC19 to obtain a PL-vFCγ p...
Embodiment 2
[0087] Example 2. Expression of recombinant dimerization proteins in stable transfected cell lines
[0088] The recombinant expression vector plasmid pCDNA3-hUTI-L-vFc γ 2 Transfection into mammalian host cell lines to express hUTI-L-vFc γ 2 Recombinant dimerized proteins. For stable high-level expression, a preferred host cell line is a DHFR enzyme-deficient CHO-cell (US Patent No. 4,818,679). A preferred method of transfection is electroporation, although other methods including calcium phosphate co-sedimentation, lipofection, and protoplast fusion can also be used. In electroporation, use a Gene Pulser Flectroporator (Bio-Rad Laboratories, Hercules, CA) set to a 250V electric field and a 960 μFd capacitance, in 2 to 5×10 cells in a cuvette. 7 Add 10 μg of plasmid DNA linearized with PvuI to each cell. Two days after transfection, the medium was changed to growth medium containing 0.6 mg / mL G418. Transfectants were screened for resistance to the selective drug using an ...
Embodiment 3
[0089] Example 3. Production of recombinant hUTI-L-vFc dimerization protein
[0090]In order to achieve high-level expression of recombinant dimerized proteins, it is advisable to use the DHFR gene inhibited by MTX drugs for co-amplification. The transfected recombinant dimerization protein gene was co-amplified with the DHFR gene in growth medium containing increasing concentrations of MTX. Transfectants capable of growing in up to 1 μg / mL MTX medium were subcloned by limiting dilution. The secretion rates of the subcloned cell lines were further analyzed. Screening for secretion levels greater than about 100 (preferably about 200) μg / 10 6 (i.e., million) cells / 24 hours of the cell line, grown in suspension in serum-free medium.
[0091] A high-yield cell strain screened by the present invention is first subjected to serum-free acclimation culture in a culture dish, and then transferred to a shaker flask for suspension acclimatization culture. Cultured in 100mL shake flas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com