Use of Prourokinase and Variants Thereof in Facilitated Percutaneous Coronary Intervention in Patients with Acute Myocardial Infarction
a technology of prourokinase and percutaneous coronary intervention, which is applied in the field of biological medicines, can solve the problems of high poor efficacy and safety of facilitated pci therapy, and patients who cannot receive direct pci therapy, so as to prevent hemorrhagic complications, reduce the incidence rate of reinfarction after thrombolysis, and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
embodiment 1
ProUK and Variants Thereof
[0028]ProUK was an extracted natural proUK or a recombinant human proUK, and had an amino acid sequence as shown in SEQ ID NO.1.
[0029]The variants of proUK were proteins or polypeptides with one or more amino acids replaced, deleted, or added in the amino acid sequence of proUK and having proUK activity; proteins with higher than 40% homology with the whole sequence of the natural proUK protein; or proteins or polypeptides with higher than 90% homology with the B chain sequence of the natural proUK protein.
[0030]A specific example of the variants of proUK was proUK having a lysine (Lys) at position 300 in the amino acid sequence of proUK as shown in SEQ ID NO.1 site-mutated into histidine (His).
[0031]ProUK and variants thereof can be prepared by a gene engineering method well-known in the art.
embodiment 2
In-Vivo and In-Vitro Experiments Indicate that a Specific Example of the Variants of proUK (Having a Lysine (Lys) at Position 300 in the Amino Acid Sequence of proUK as Shown in SEQ Id NO.1 Site-Mutated into Histidine (his), Lys300 →His, M5) has a Thrombolytic Activity at Least Equivalent to or Superior to that of proUK, and Thus is Applicable in Facilitated PCI Therapy
[0032](1) In-vitro Experimental Results of M5
[0033]1.1 Intrinsic Catalytic Activity Test
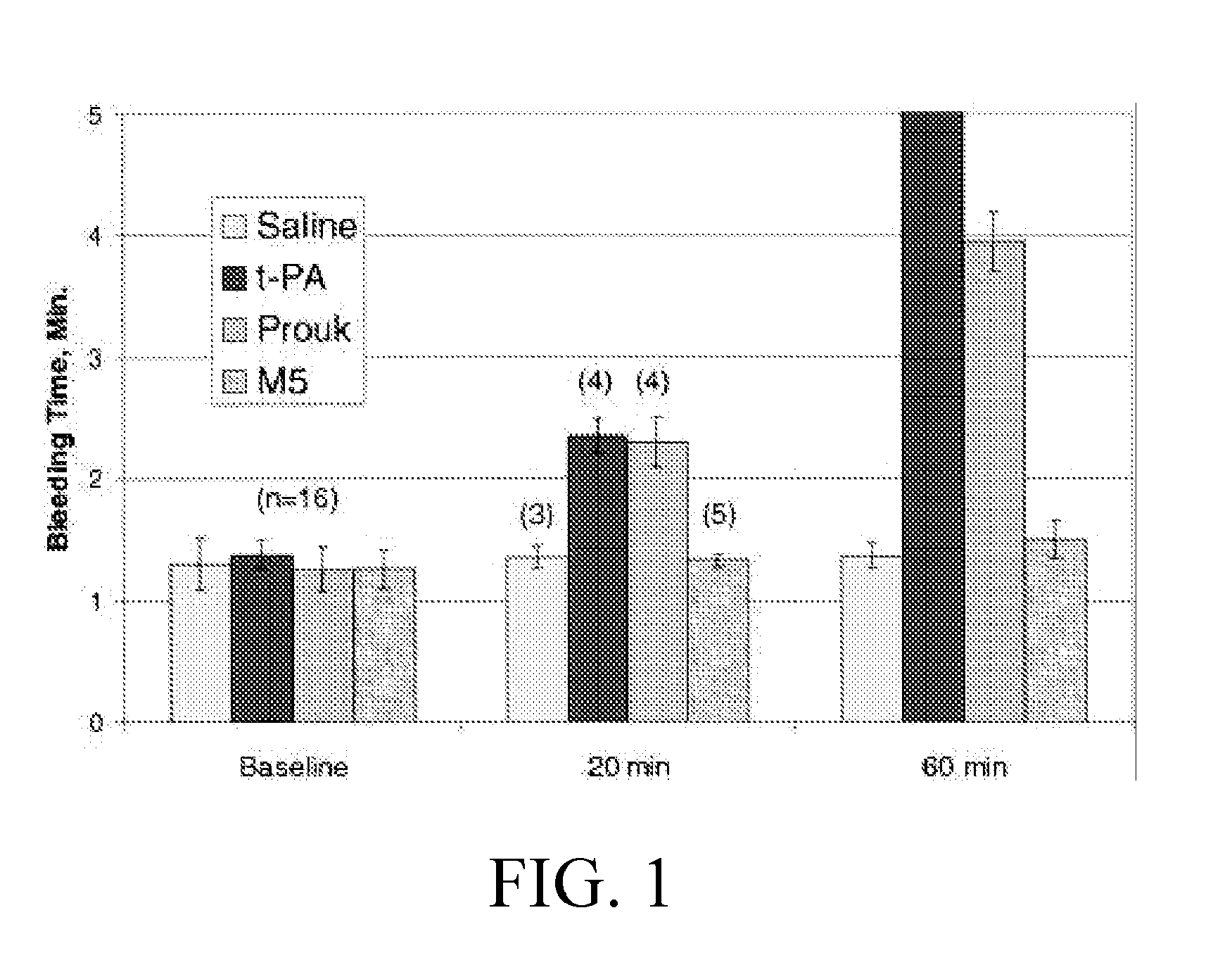
[0034]As for the hydrolysis of a chromophoric substrate S2444 (L-pGlu-L-Gly-Arg p-nitroanilide hydrochloride), 1.0 mol / L proUK or 100 mol / L M5 and a series of concentrations (0-2.4 mmol / L) of S2444 were co-incubated in a buffer solution (0.05 mol / L Tris-HCl, 0.10 mol / L NaCl, and 0.01% Tween 80, pH 7.4) at room temperature, the reaction rate was determined by an increment in light absorption at 410 nm, and the reaction constant was calculated through a Lineweaver-Burk plot. The results show that, the lag phase of MS is twice that of...
embodiment 3
Application of ProUK in Facilitated PCI
[0055](1) Selection Criteria
[0056]1.1 Ischemic chest pain lasting for 30 min or more, and sublingual administration of nitroglycerin being ineffective;
[0057]1.2 Sustained ischemic chest pain lasting for 12 hrs or less;
[0058]1.3 Electrocardiogram (ECG) having at least two or more ST segment elevations of 0.1 mV or more in limb lead, or two or more adjacent ST segment elevations of 0.2 mV or more in chest lead; and
[0059]1.4 Age of 85 years old or less, male or female.
[0060](2) Exclusion Criterion
[0061]2.1 Non-ST segment-elevation AMI or unstable angina pectoris;
[0062]2.2 Women during pregnant stage, breast-feed stage, and menstrual period;
[0063]2.3 Hemorrhagic stroke occurred at anytime in the past, and ischemic stroke or cerebrovascular event occurred in 1 year;
[0064]2.4 Severe progressive diseases (such as malignant tumor) or diseases with poor prognosis and making the patient to be extremely exhausted;
[0065]2.5 Active visceral hemorrhage (such...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com