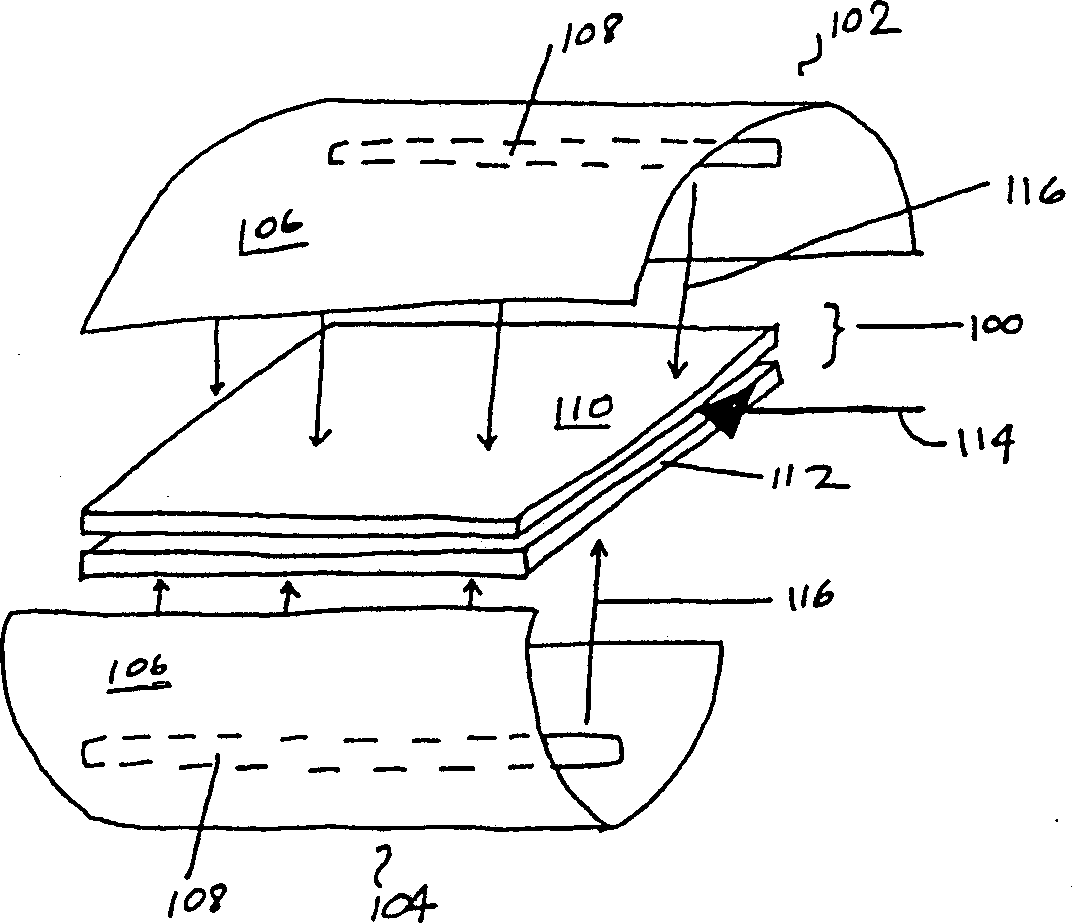
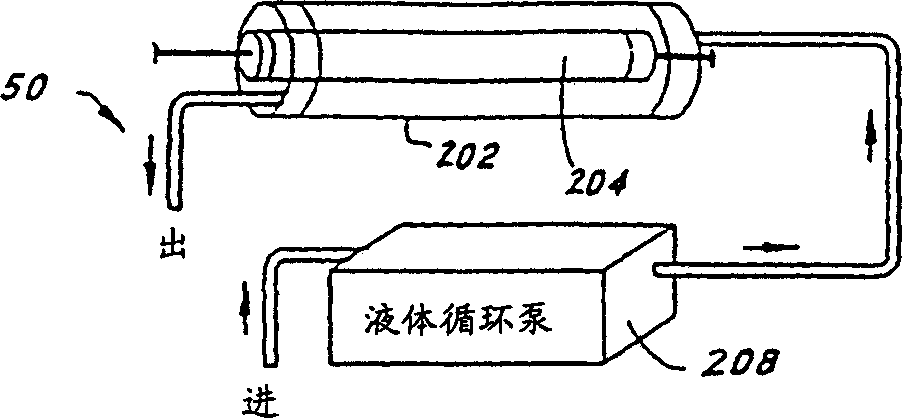
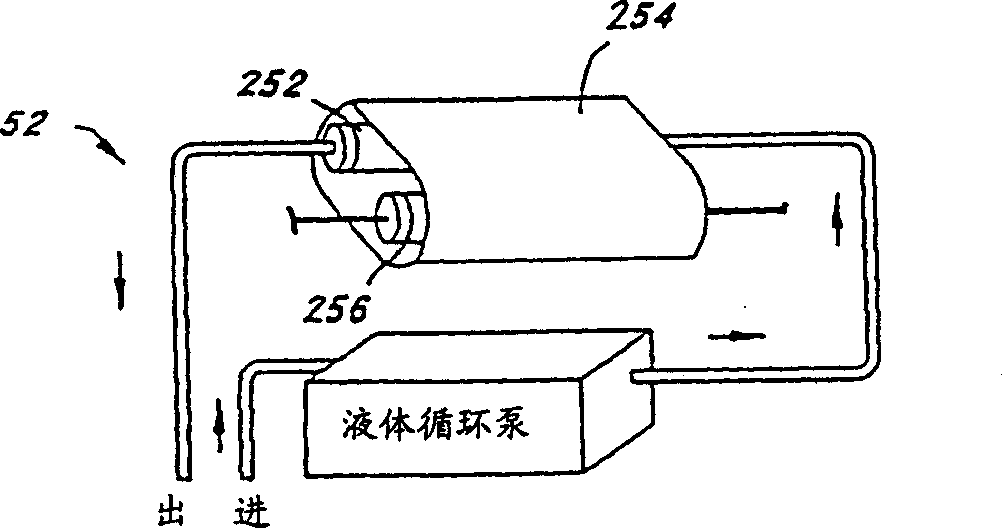
Methods for inactivating pathogens using broad-spectrum pulsed light
A pathogen and pulse technology, applied in the field of inactivating pathogens with broad-spectrum pulsed light, can solve the problems such as BPL not raised
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Example 1: Inactivation of SV40
[0059] African green monkey kidney (AGMK) cells were inoculated with simian virus 40 (SV40, ATCC VR-305). Virus was harvested when 75%-100% of cells exhibited cytopathic effect (CPE). The virus stocks were frozen and stored at -60°C in culture medium containing 10%-15% fetal bovine serum (FBS). SV40-containing samples were titrated with AGMK cells. The culture medium used to grow and maintain AGMK cells is Eagles minimal essential medium (E-MEM), containing 5%-10% heat-inactivated FBS, 10 mg / ml gentamicin, 100 units / ml penicillin, and 2.5 mg / ml amphotericin B.
[0060] Cell cultures were grown at 36-38°C in a humidified 5-7% CO2 atmosphere.
[0061] SV40 in E-MEM containing 10% heat-inactivated FBS was added in a volume of 0.2 ml to sterile polyethylene sample containers. Twelve samples were prepared. Nine samples were treated with BPL. Three samples were treated with one pulse of light, three samples were treated with two pulses...
Embodiment 2
[0065] Example 2: Inactivation of HIV-1
[0066] CCRF-CEM cells were inoculated with HIV-type I strain HTLVIIIB (Vanderbilt University, Nashville, TN). HIV-1 was collected when the infectivity reached a minimum of 80% as determined by immunofluorescence assay (IFA). Frozen virus stocks, stored at -60°C in culture medium containing 10%-15% FBS.
[0067] HIV-1 containing samples were titrated with MT-2 cells (NIH AIDS Research and Reference Reagent Program. Cat #237). The medium used for the growth and maintenance of MT-2 cells was supplemented with 2 mM L-glutamine, 25 mM Hepes, 2 g / L NaHCO 3 , RPMI 1640 (with phenol red) in 15% heat-inactivated FBS and 50 mg / ml gentamycin. at 36-38°C in humidified 5-7% CO 2 Cell cultures were grown in the atmosphere.
[0068] 0.2 ml of HIV formulated in RPMI containing 15% heat-inactivated FBS was added to a sterile polyethylene sample container. Twelve samples were prepared. Nine samples were treated with BPL. Among them, three copies...
Embodiment 3
[0073] Example 3: Inactivation of CPV
[0074] A-72 cells (ATCC CRL 1542) were inoculated with CPV (ATCCVR-2017). Virus was harvested when 75%-100% of the cells showed cytopathic effect (CPE). Frozen virus stocks, stored at -60°C in culture medium containing 10%-15% FBS.
[0075]CPV-containing samples were titrated with A-72 cells. The medium used for the growth and maintenance of A-72 cells was E supplemented with 5%-10% heat-inactivated FBS, 10 mg / ml gentamicin, 100 units / ml penicillin, and 2.5 mg / ml amphotericin B. -MEM. Humidified 5-7% CO at 36-38°C 2 Cell cultures were grown in the atmosphere.
[0076] 0.2 ml of CPV in E-MEM containing 5% heat-inactivated FBS was added to a sterile polyethylene sample container. Twelve samples were prepared. Nine samples were treated with BPL. Among them, three copies were treated with one light pulse, three copies were treated with two pulses, and three copies were treated with three pulses. Three untreated samples served as con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com