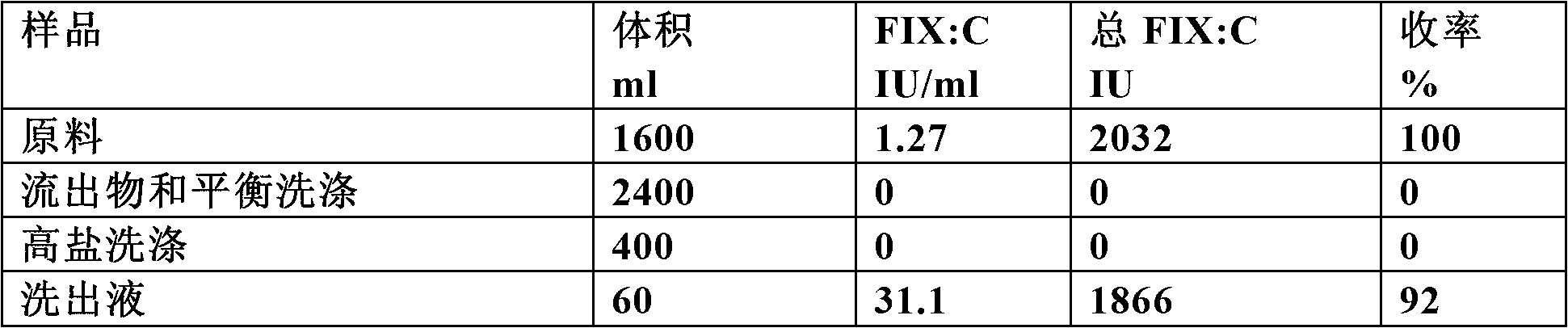
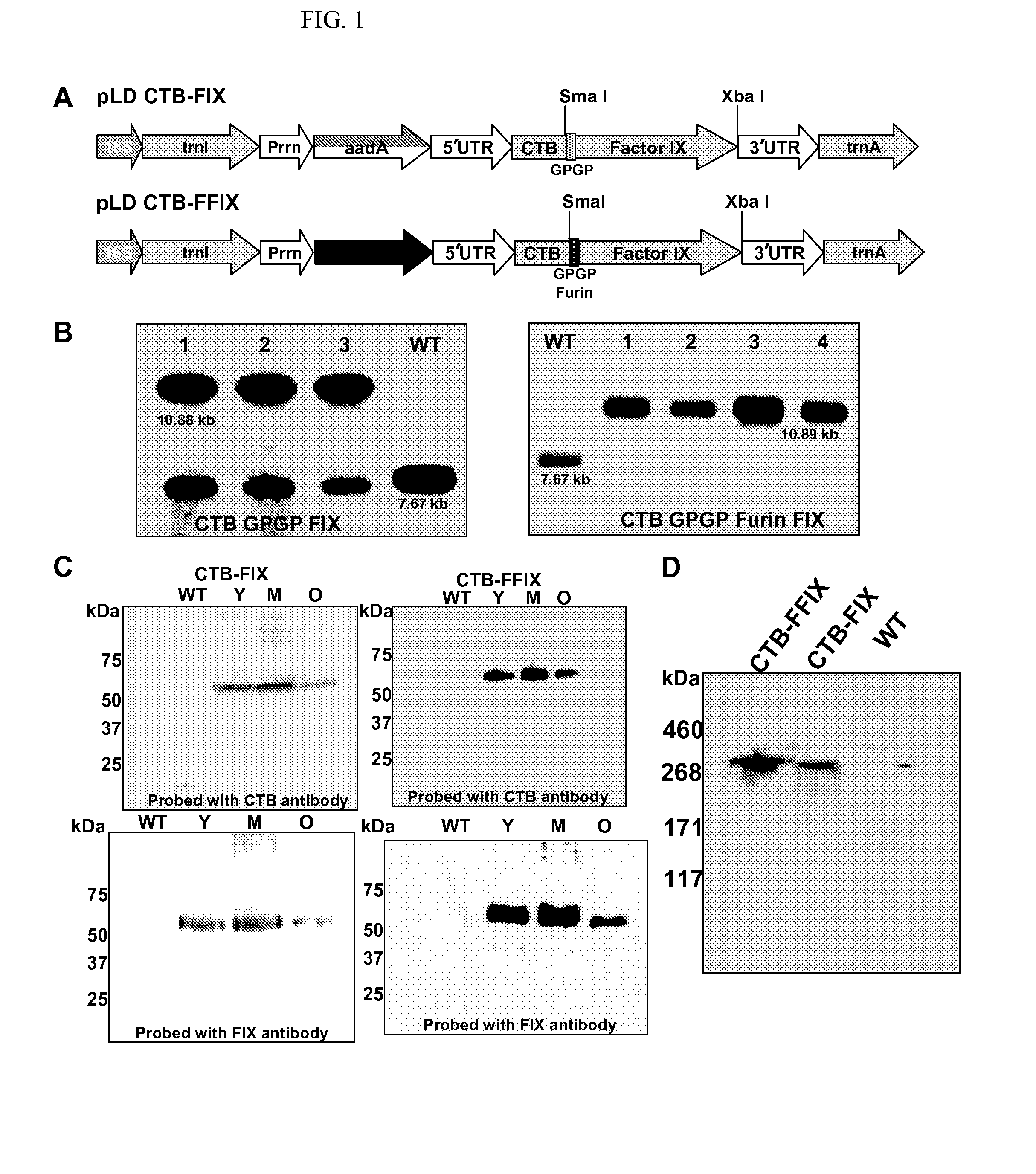
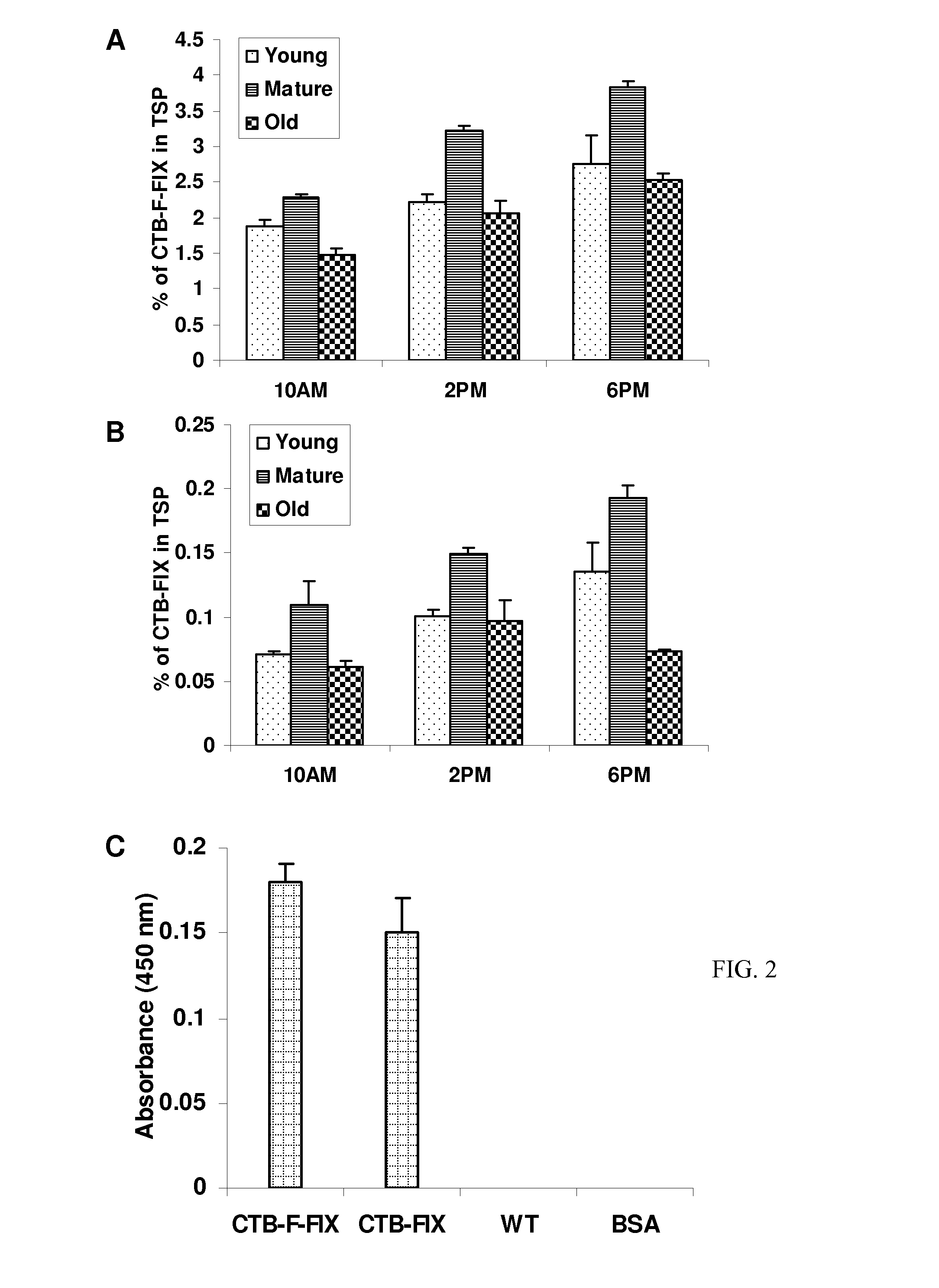
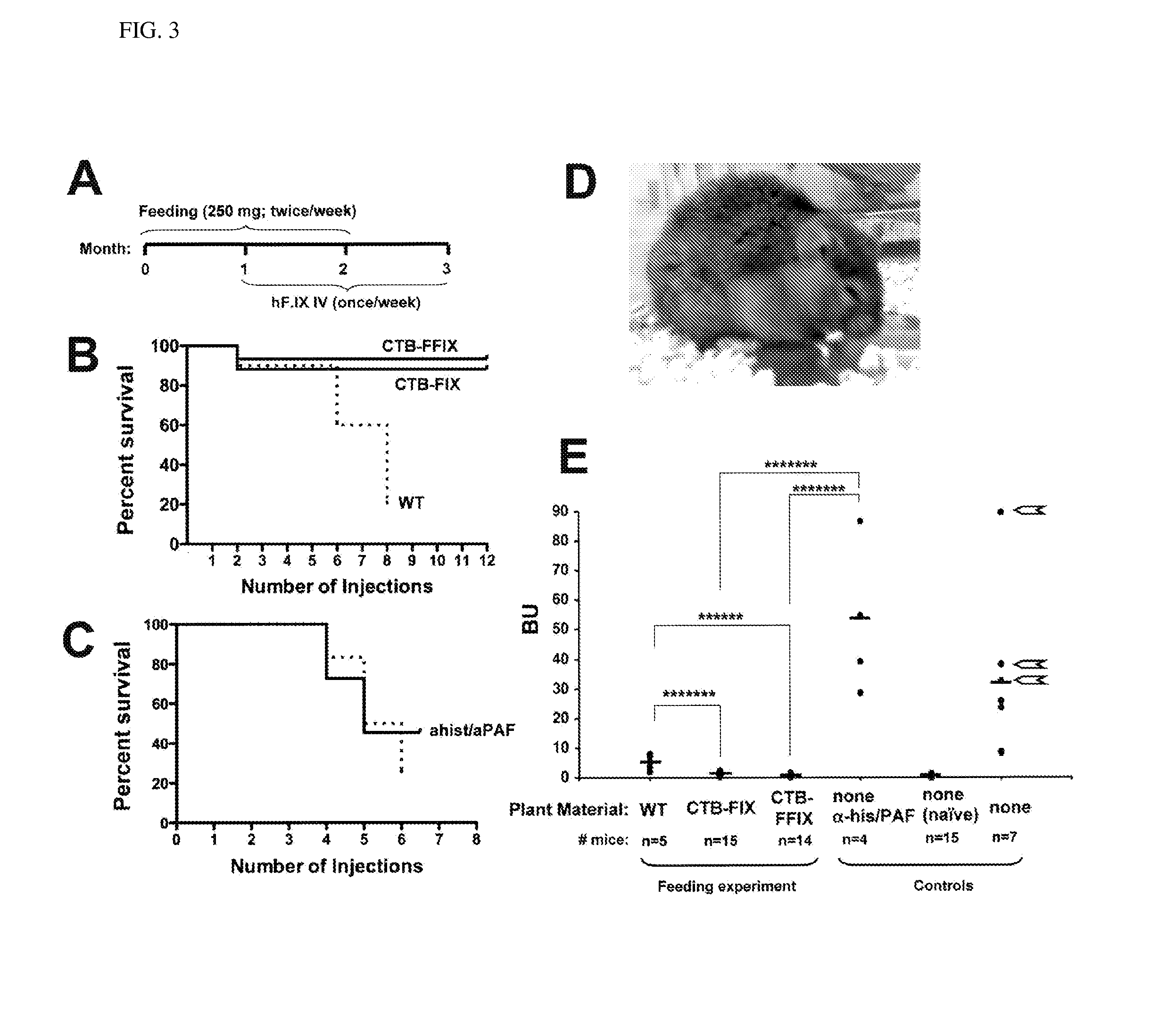
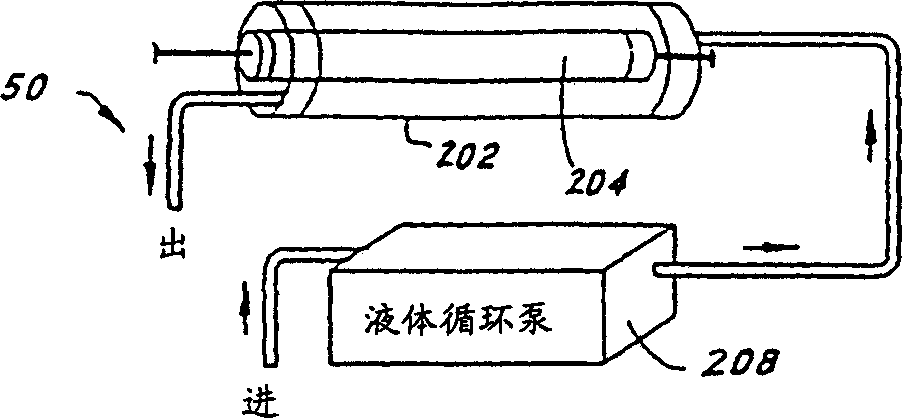
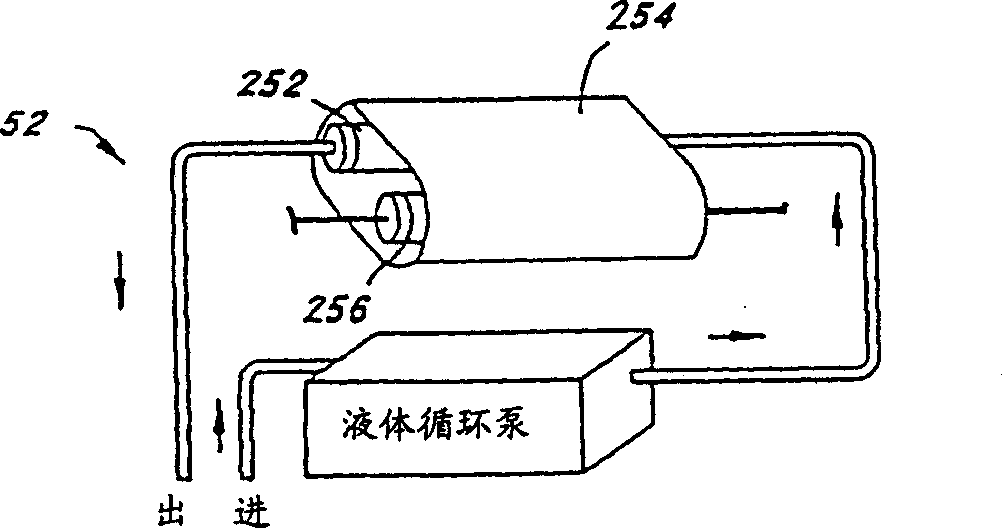
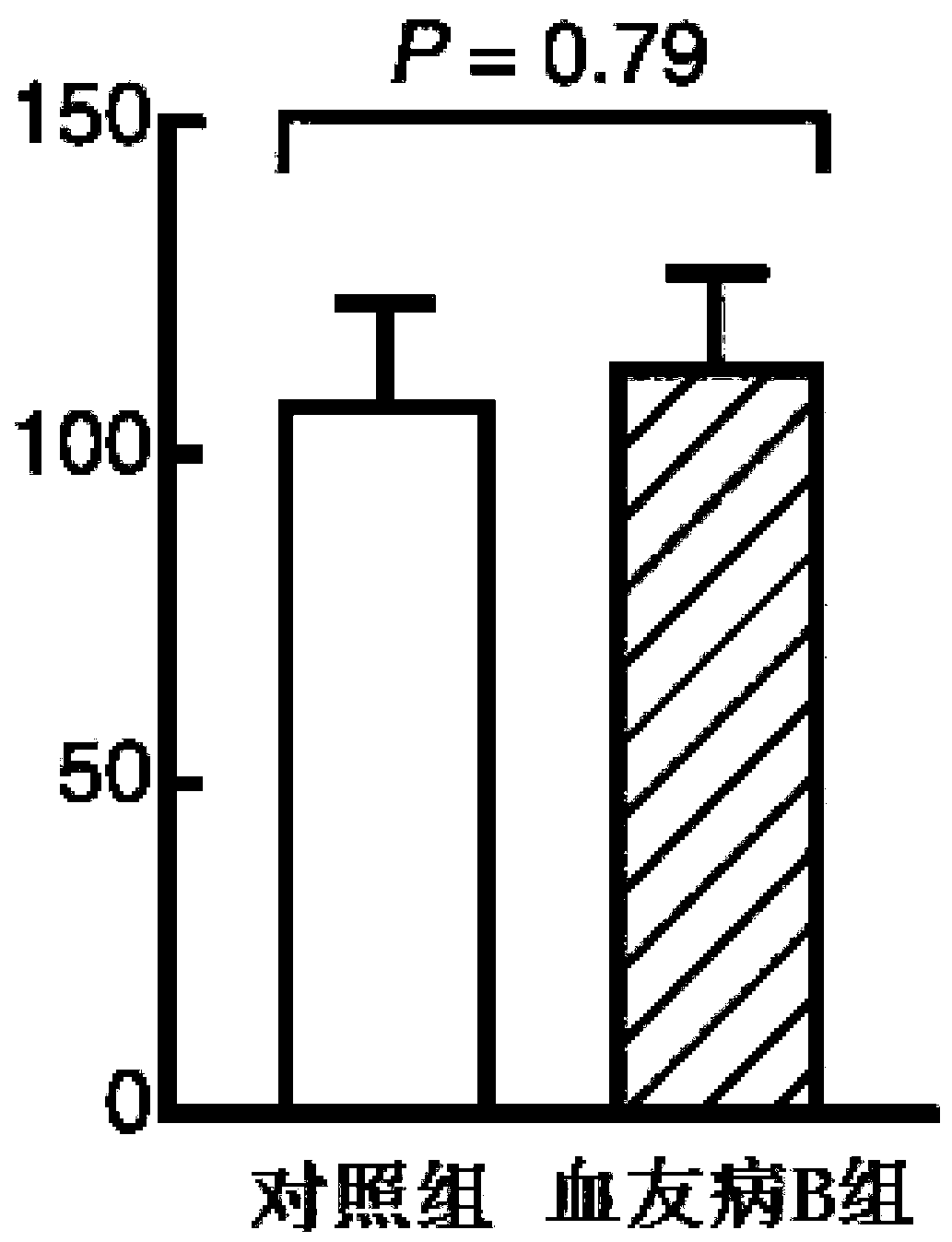
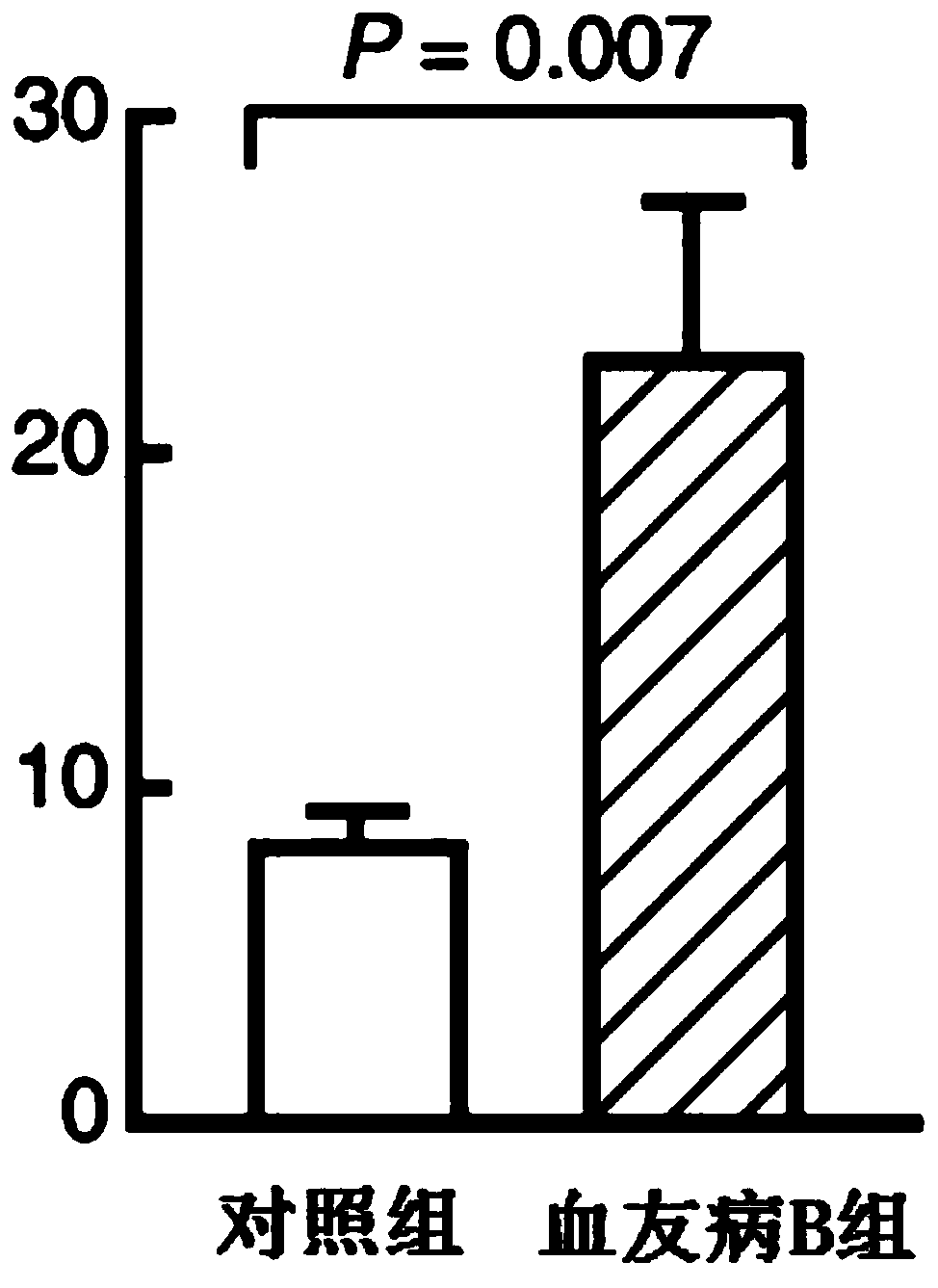
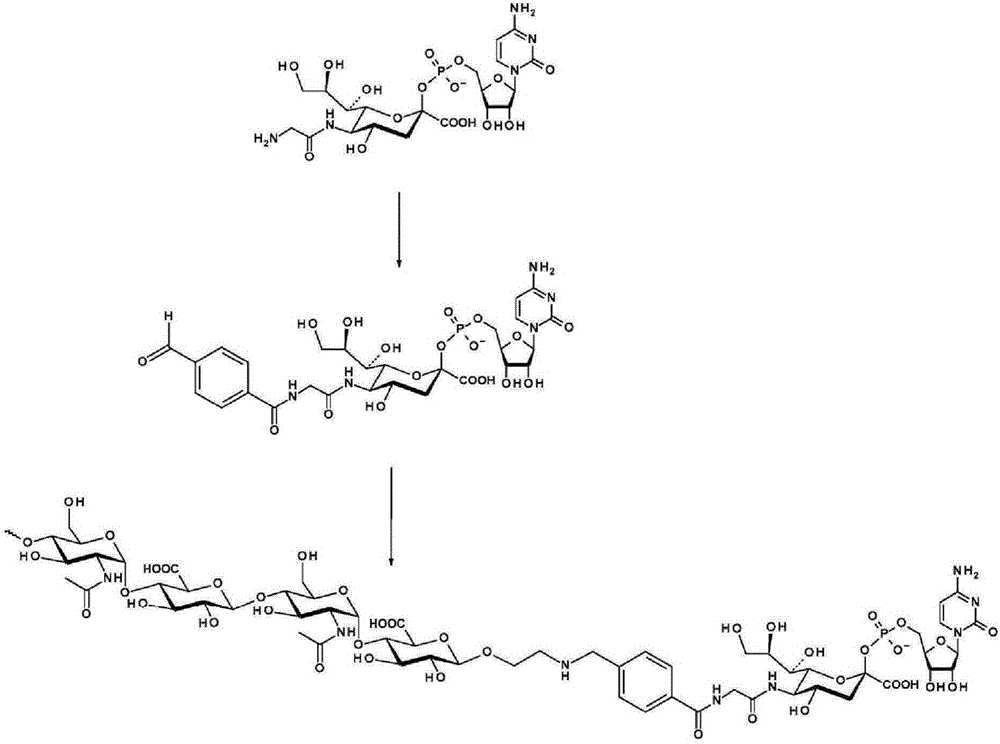
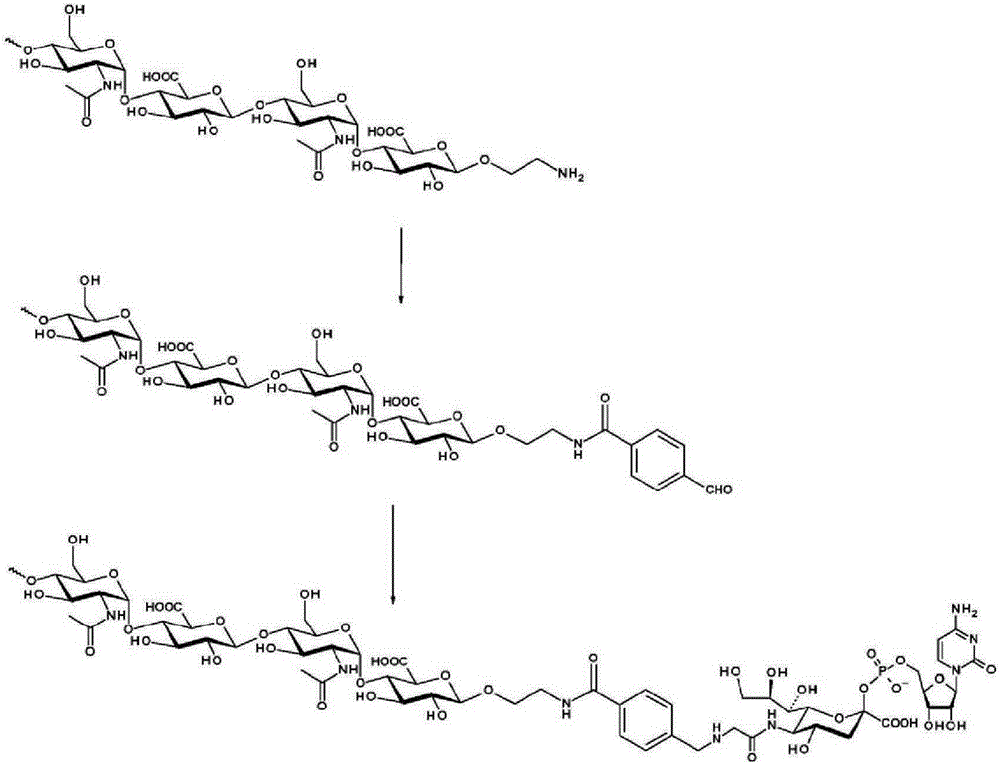
Patents
Literature
38 results about "Coagulation Factor IX" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
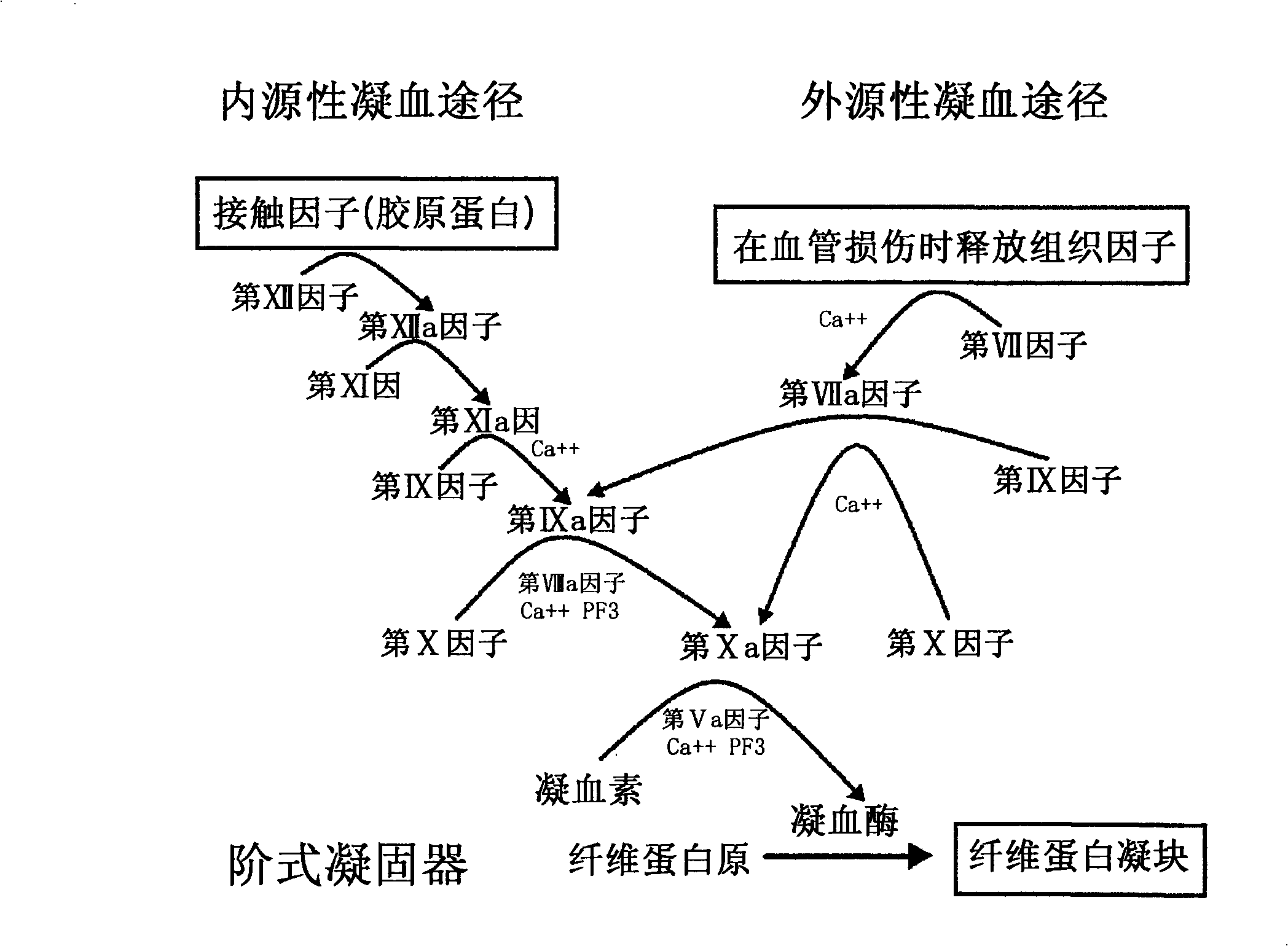
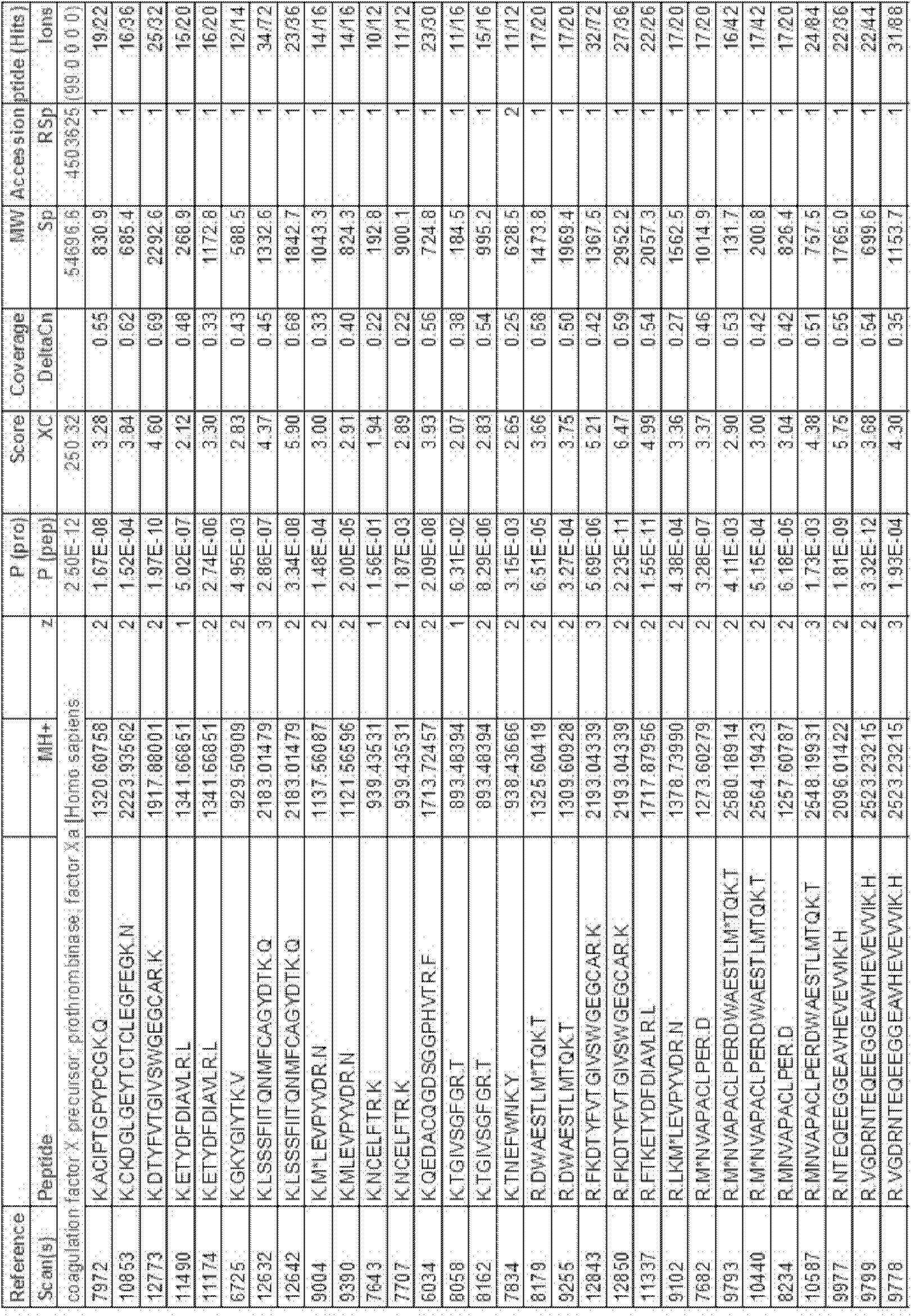
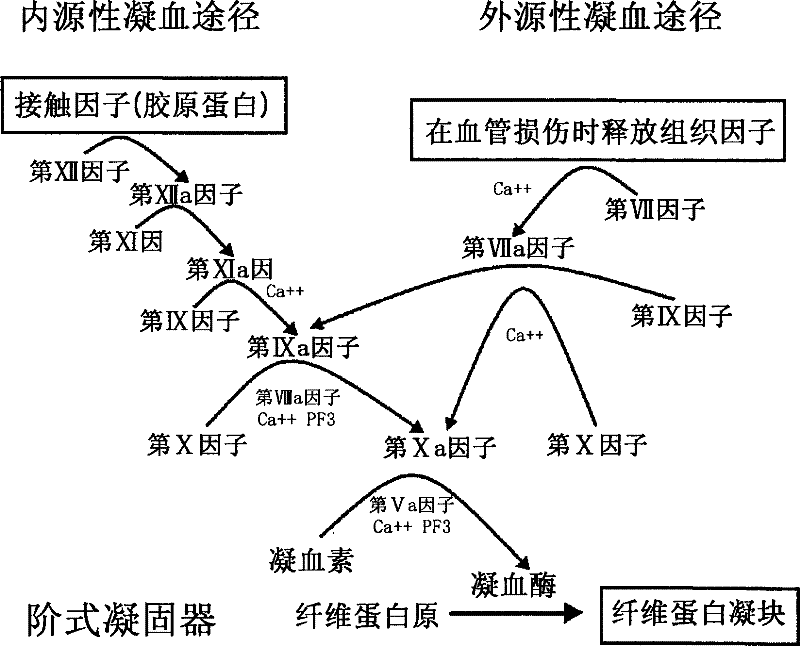
Coagulation factor IX (461 aa, ~52 kDa) is encoded by the human F9 gene. This protein plays a role in the intrinsic pathway of blood coagulation through the mediation of proteolysis that converts factor X into an active protease.
Preparation method of high-purity human coagulation factor IX
InactiveCN105175486AReduce lossesPrevent denaturation and inactivationPeptide preparation methodsUltrafiltrationPolyethylene glycol
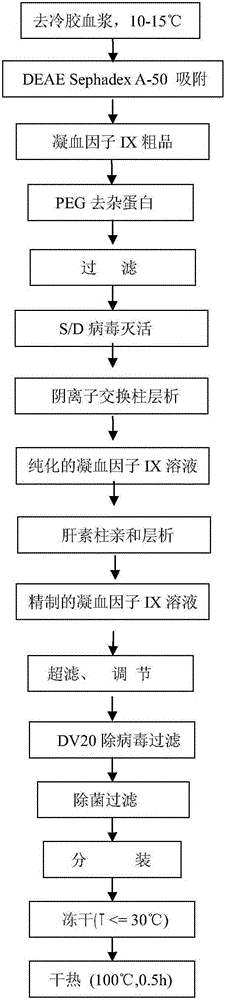
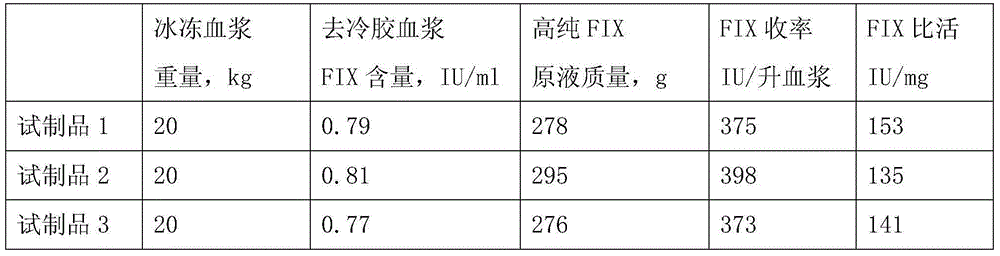
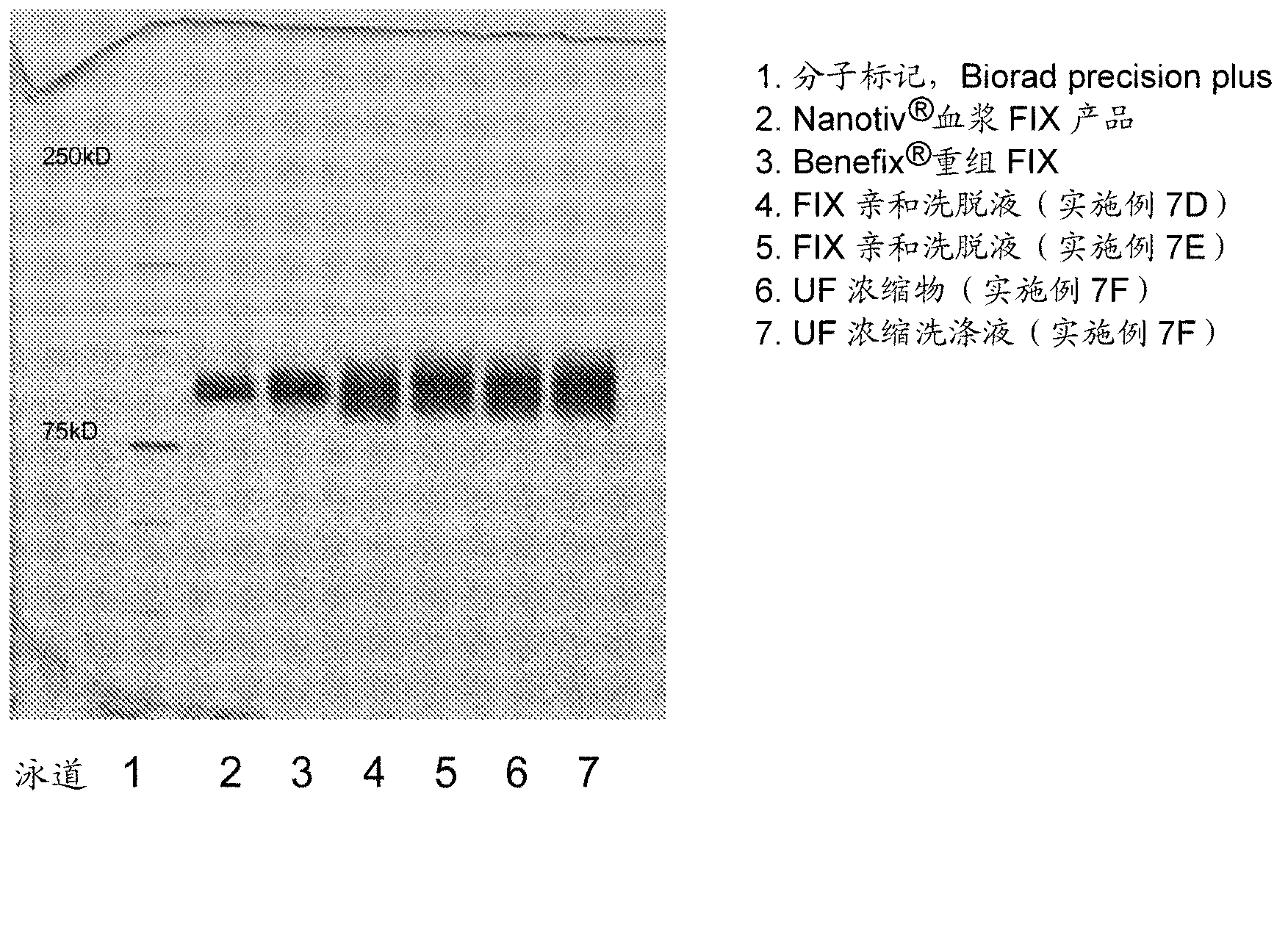
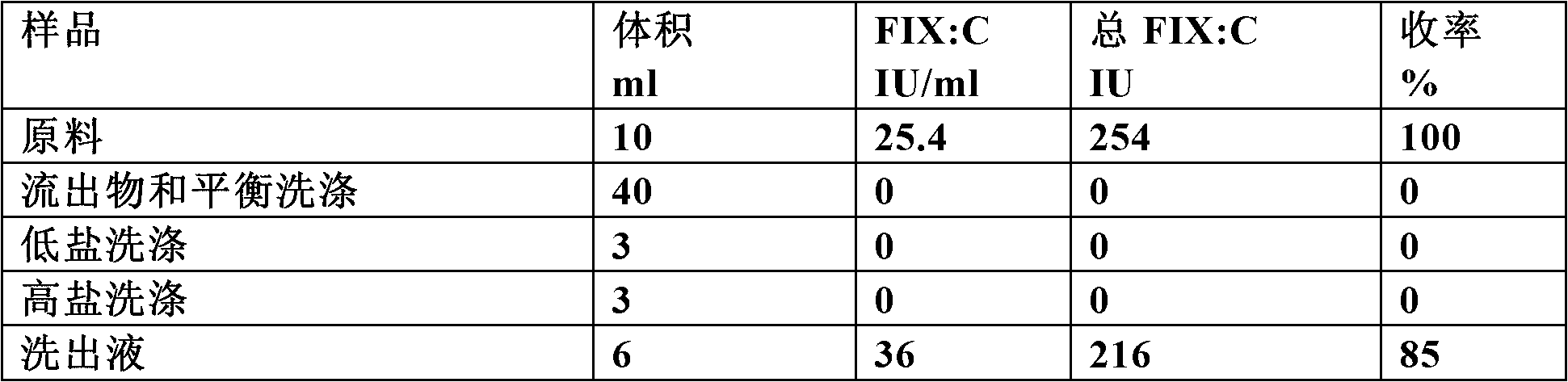
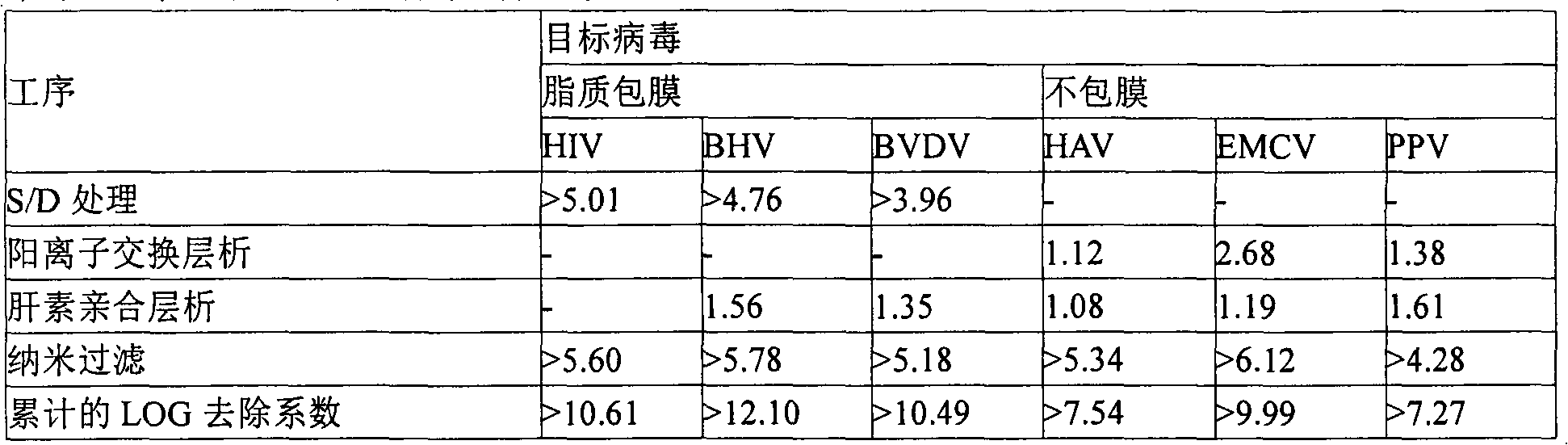
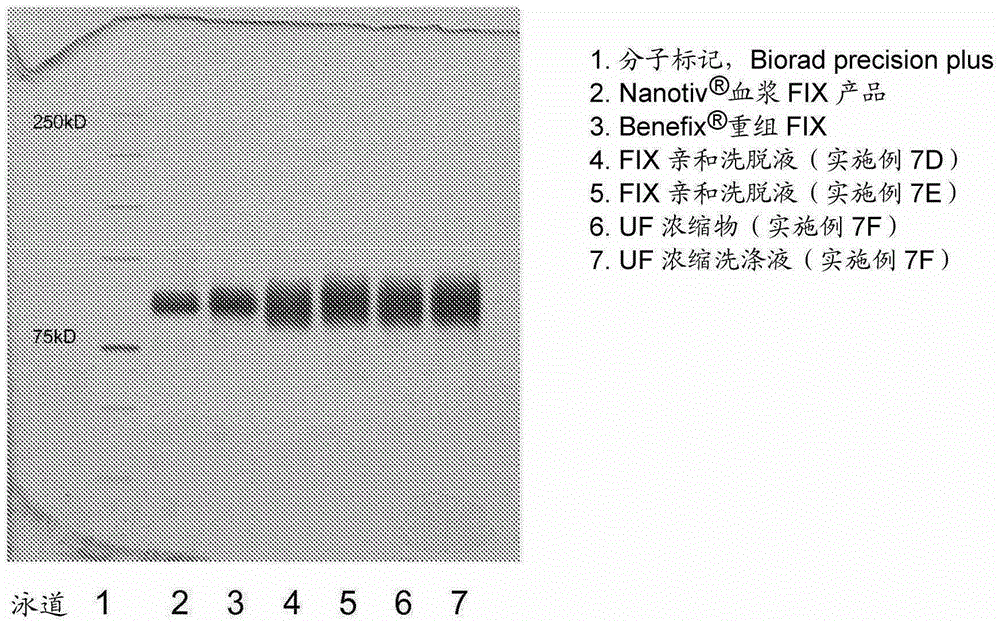
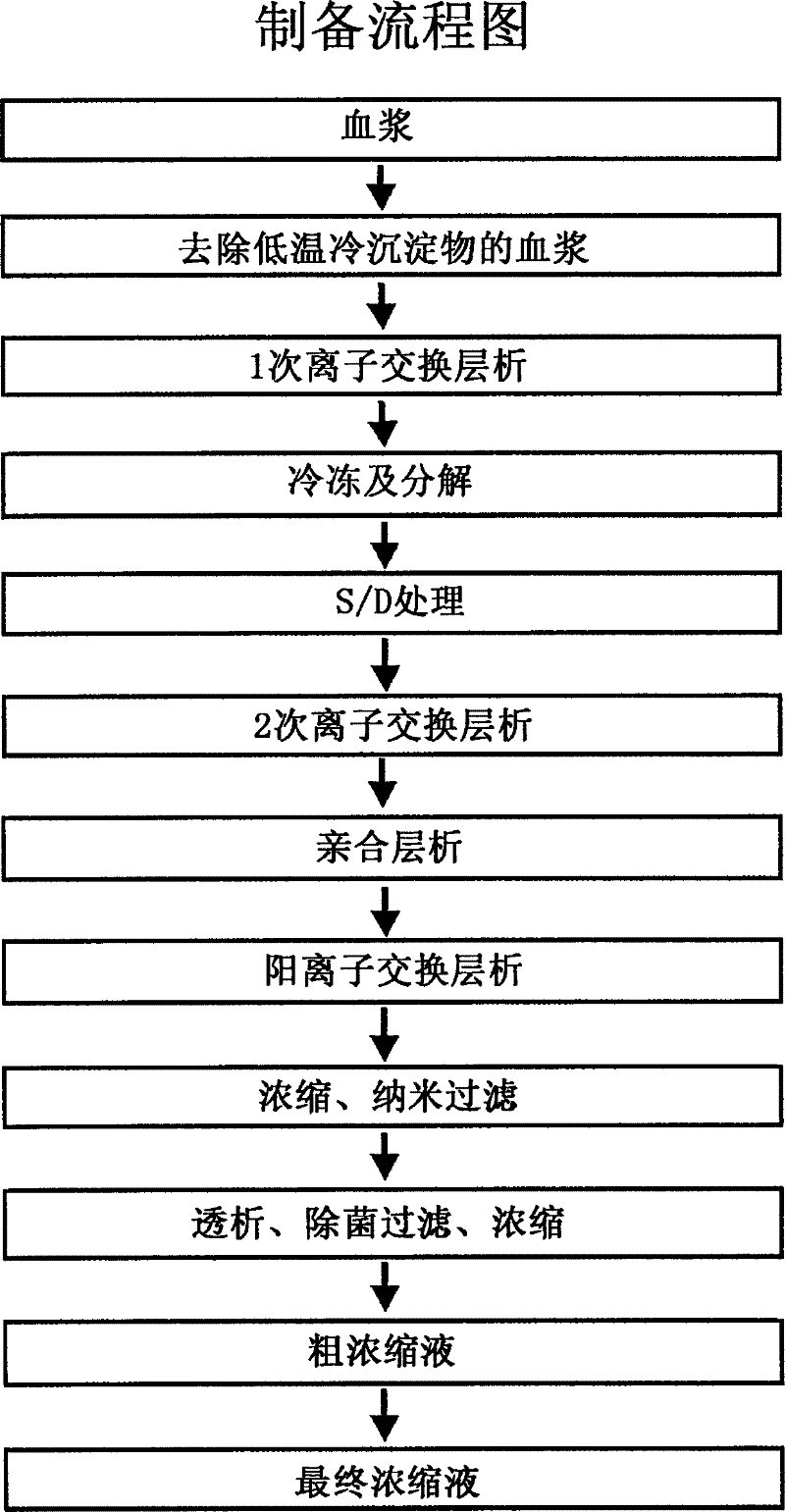
The invention relates to a preparation method of a high-purity human coagulation factor IX, which comprises the following steps: melting refrigerated plasma, and carrying out low-temperature centrifugation; adsorbing with a DEAE (diethylaminoethanol) Sephadex A-50 gel to remove the coagulation factor IX in the cold-glue plasma; removing impure proteins in the solution by using polyethyleneglycol; carrying out S / D virus inactivation; carrying out anion exchange column chromatography to obtain a purified coagulation factor IX solution; passing through a heparin affinity column for further chromatography to obtain a high-purity coagulation factor IX solution; carrying out ultrafiltration, dialysis and concentration, and adding arginine hydrochloride and glycinate as protective agents; filtering through a 20nm filter element to remove viruses; carrying out freeze-drying; and carrying out dry heat virus inactivation. The protein protective agents are added during the gel adsorption, column chromatography and ultrafiltration dialysis, thereby lowering the activation probability of the FIX product thrombin and enhancing the qualification rate of the product. The technique has high product yield; the FIX specific activity can reach 150 IU / mg or so which is much higher than that of the traditional product; and by performing the three-step virus inactivation, the product is safe and reliable to use.
Owner:上海洲跃生物科技有限公司
A process for purifying vitamin k dependent proteins such as coagulation factor IX
InactiveCN102858971AGuarantee structureHydrolasesPeptide preparation methodsArginineAqueous solution
Owner:OCTAPHARMA
Administration of plant expressed oral tolerance agents
Protein replacement therapy for patients with hemophilia or other inherited protein deficiencies is often complicated by pathogenic antibody responses, including antibodies that neutralize the therapeutic protein or that predispose to potentially life-threatening anaphylactic reactions by formation of IgE. Using murine hemophilia B as a model, we have developed a prophylactic protocol against such responses that is non-invasive and does not include immune suppression or genetic manipulation of the patient's cells. Oral delivery of coagulation factor IX (F. IX) expressed in chloroplasts, bioencapsulated in plant cells, effectively blocked formation of inhibitory antibodies in protein replacement therapy. Inhibitor titers were mostly undetectable and up to 100-fold lower in treated mice when compared to controls. Moreover, this treatment eliminated fatal anaphylactic reactions that occurred after 4 to 6 exposures to intravenous F. IX protein. While only 20-25% of control animals survived after 6-8 F. IX doses, 90-95% of tolerized mice survived 12 injections without signs of allergy or anaphylaxis. This high-responder strain of hemophilia B mice represents the first hemophilic animal model to study anaphylactic reactions. The plant material was effective over a range of oral antigen doses (equivalent to 5-80 μg recombinant F.IX / kg), and controlled inhibitor formation and anaphylaxis long-term, up to 7 months. Oral antigen administration caused a deviant immune response that suppressed formation of IgE and inhibitory antibodies. This cost-effective and efficient approach to oral delivery of protein antigens to the gut should be applicable to several genetic diseases that are prone to pathogenic antibody responses during treatment.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
Fusion protein having factor ix activity
Disclosed is a fusion protein comprising blood coagulation factor IX (FIX) and transferrin. The fusion protein exhibits improved specific FIX activity, as compared to native FIX, and can be useful in the treatment of FIX deficiency-associated diseases.
Owner:TIUMBIO CO LTD
Method for manufacturing high purified factor ó¨
ActiveCN101291951APeptide preparation methodsPeptidasesCell culture mediaAnion-exchange chromatography
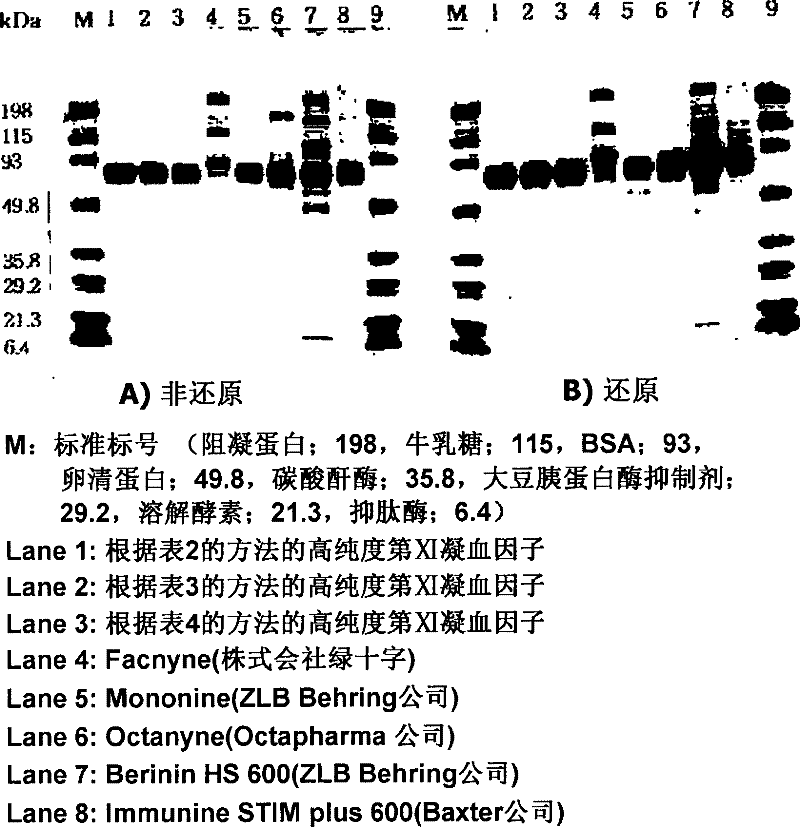
The present invention discloses a preparation method of a highly purified human blood coagulation factor IX. The human blood coagulation factor IX is prepared by performing anion exchange chromatography, cation exchange chromatography, heparin affinity chromatography to the material containing human blood coagulation factor IX (taken from human blood serum or recombined cell culture medium), which includes the period of passivating virus through S / D treatment (Solvent / Detergent treatment) or removing virus through nanofiltration. The highly purified safe coagulation factor IX preparation having a specific activity of above 150 IU / mg, with substantially undoped proteins can be prepared by the preparation method of the invention.
Owner:THE GREEN CROSS CORP
Method for efficiently extracting and purifying blood coagulation factor IX and blood coagulation factor X
InactiveCN102146134AHigh affinityImprove stabilityPeptide preparation methodsBlood coagulation/fibrinolysis factorsBlood Coagulation Factor XBlood plasma
The invention provides a method for efficiently extracting and purifying a blood coagulation factor IX and a blood coagulation factor X. The method provided by the invention comprises the following steps: collecting blood, treating, and collecting blood plasma; and extracting an IX crude extract and an X crude extract from the blood plasma, and purifying. The method is characterized in that the purifying step is to use FIX / FX-bp-Sepharose 4B to purify the blood coagulation factor IX crude extract and the blood coagulation factor X crude extract respectively through the affinity chromatography; and the affinity ligand FIX / FX-bp is selected from ACF I, ACF II or AHP. The purified blood coagulation factor IX can be directly used as a medicament and the purified blood coagulation factor X canbe directly used as a reagent.
Owner:UNIV OF SCI & TECH OF CHINA
Antithrombotic agents
InactiveCN1501812AImmunoglobulins against blood coagulation factorsPeptide/protein ingredientsAntithrombotic AgentMonoclonal antibody
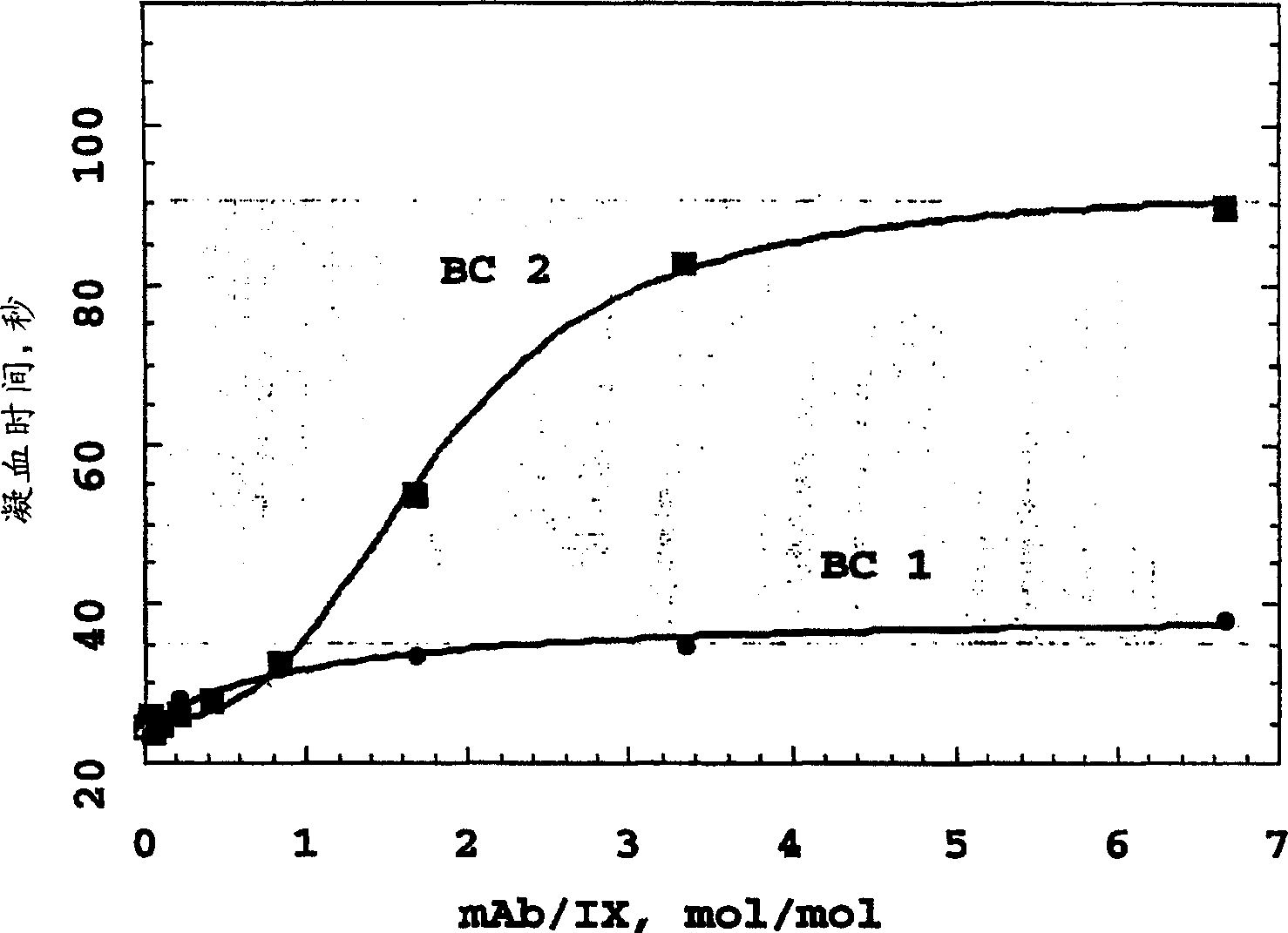
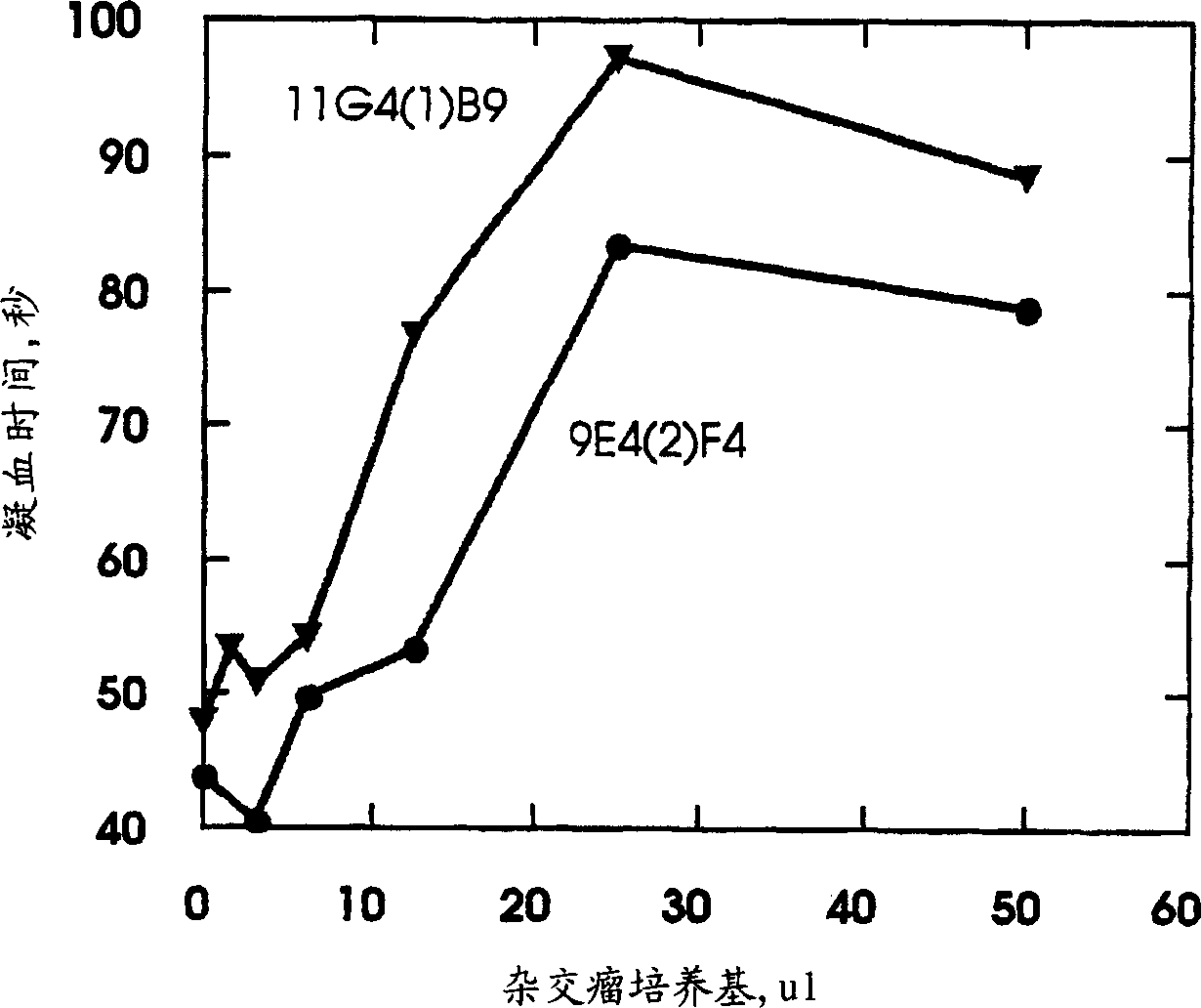
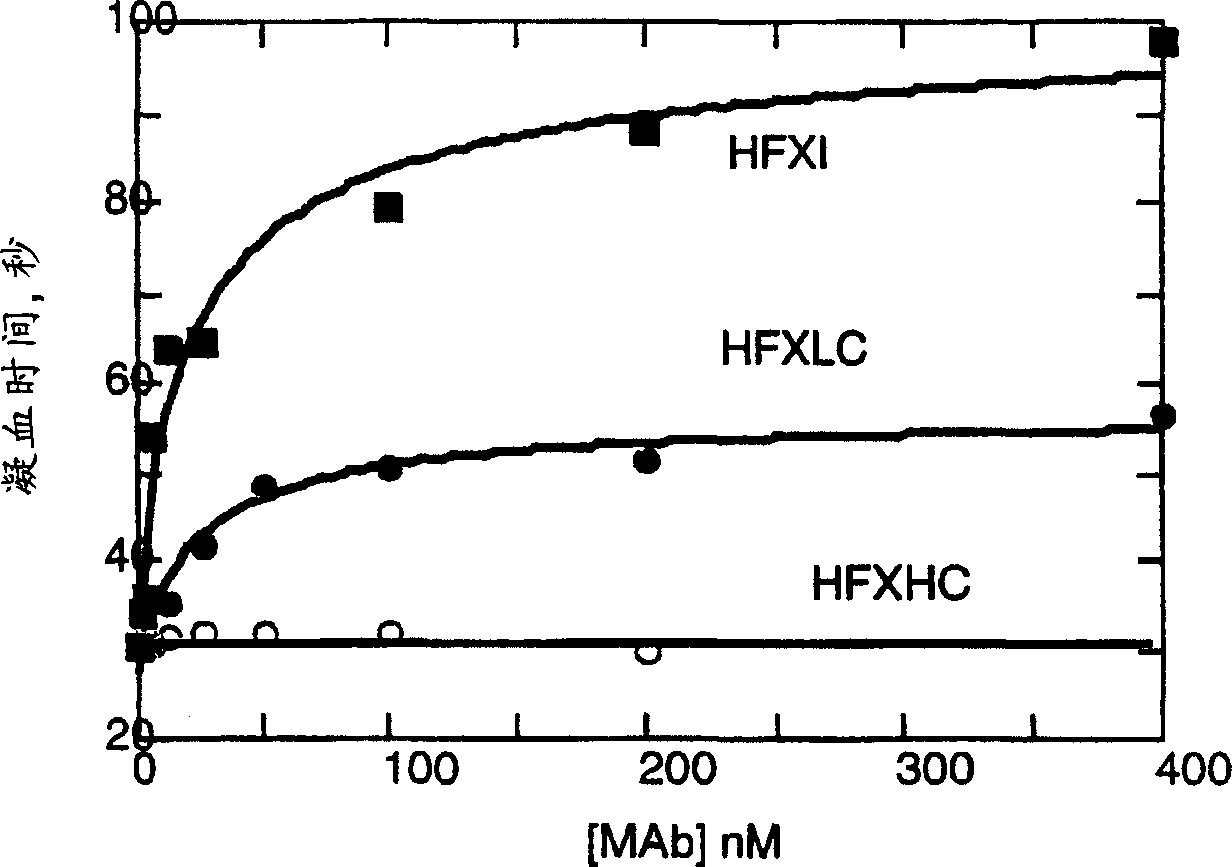
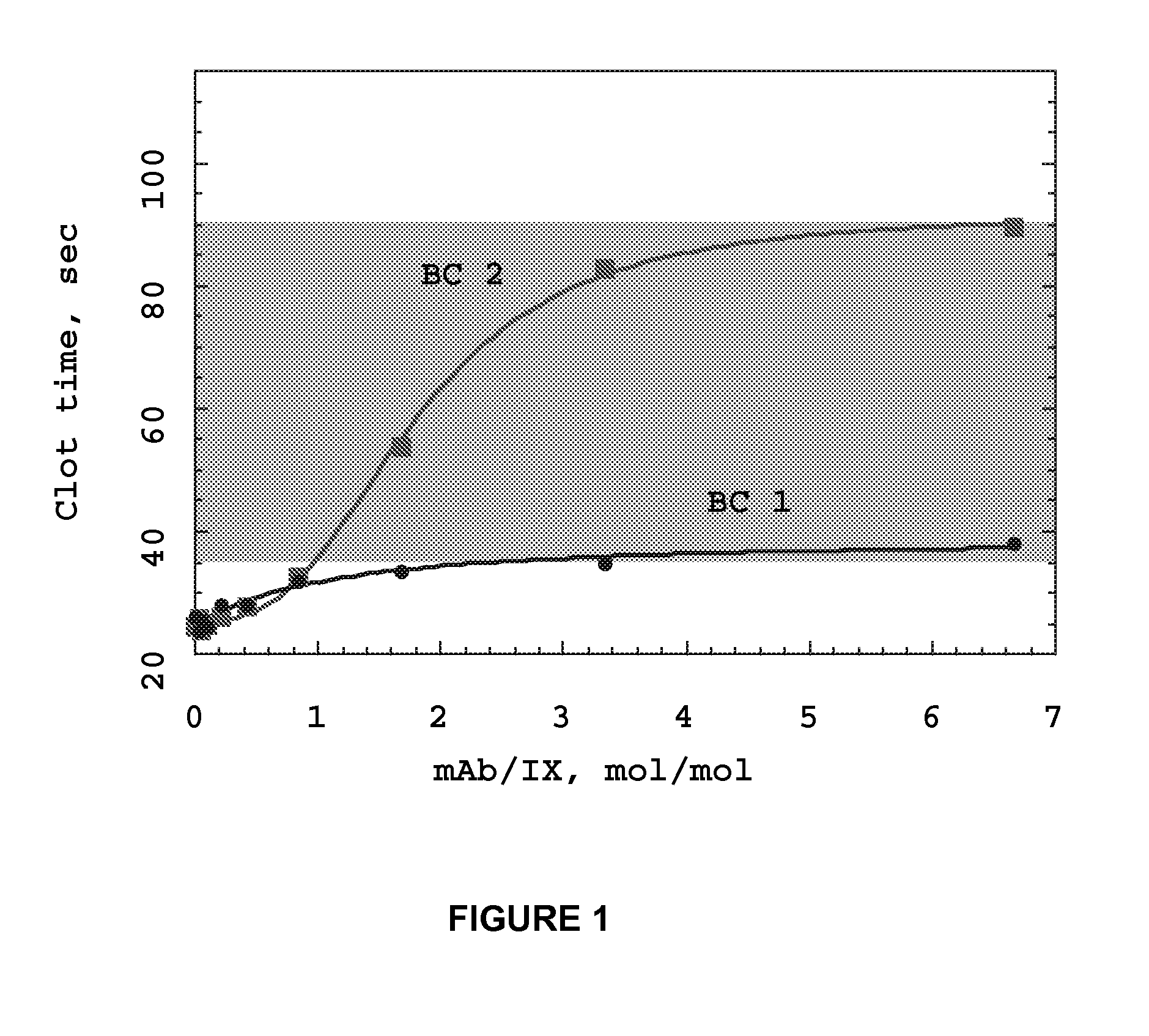
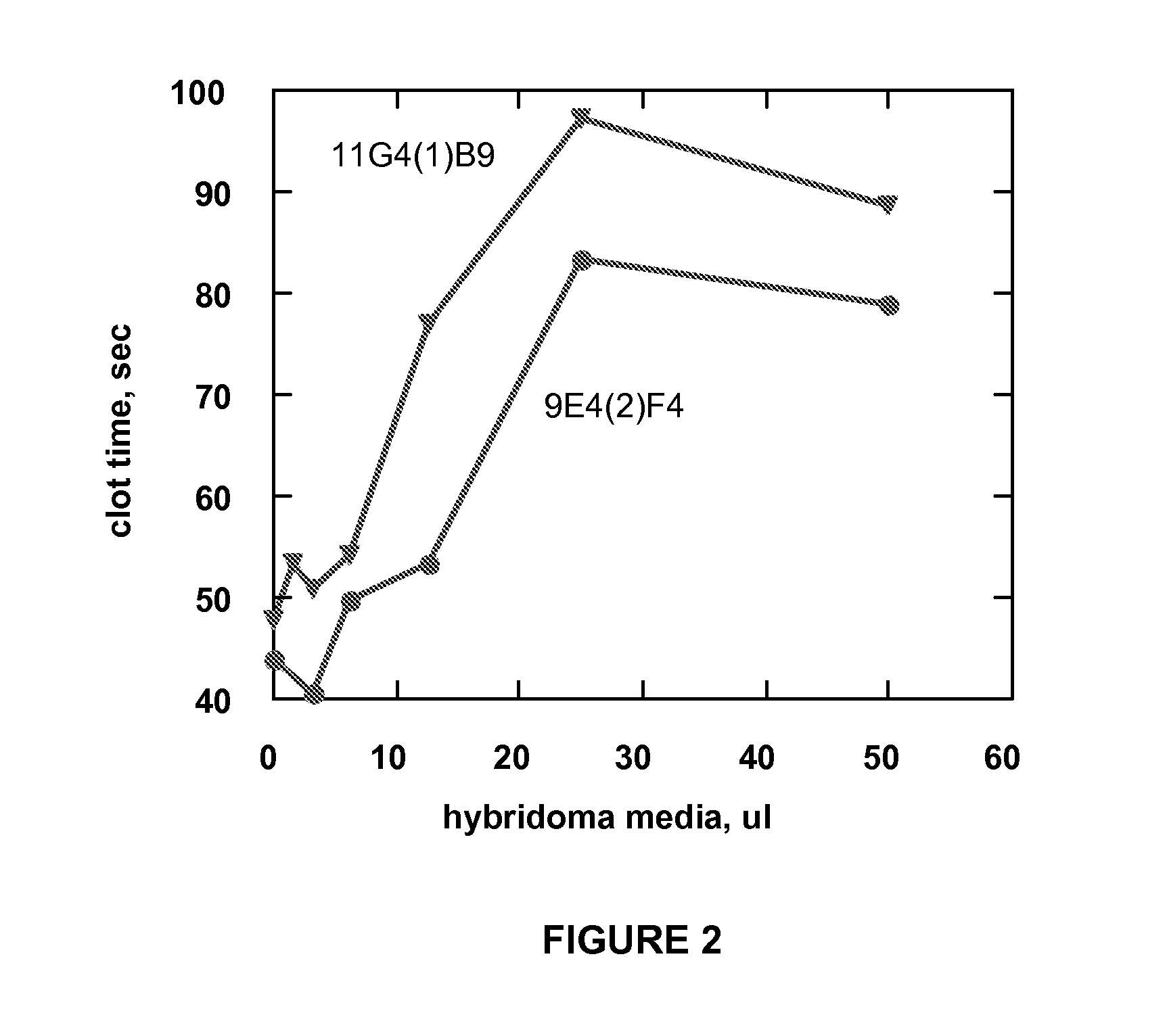
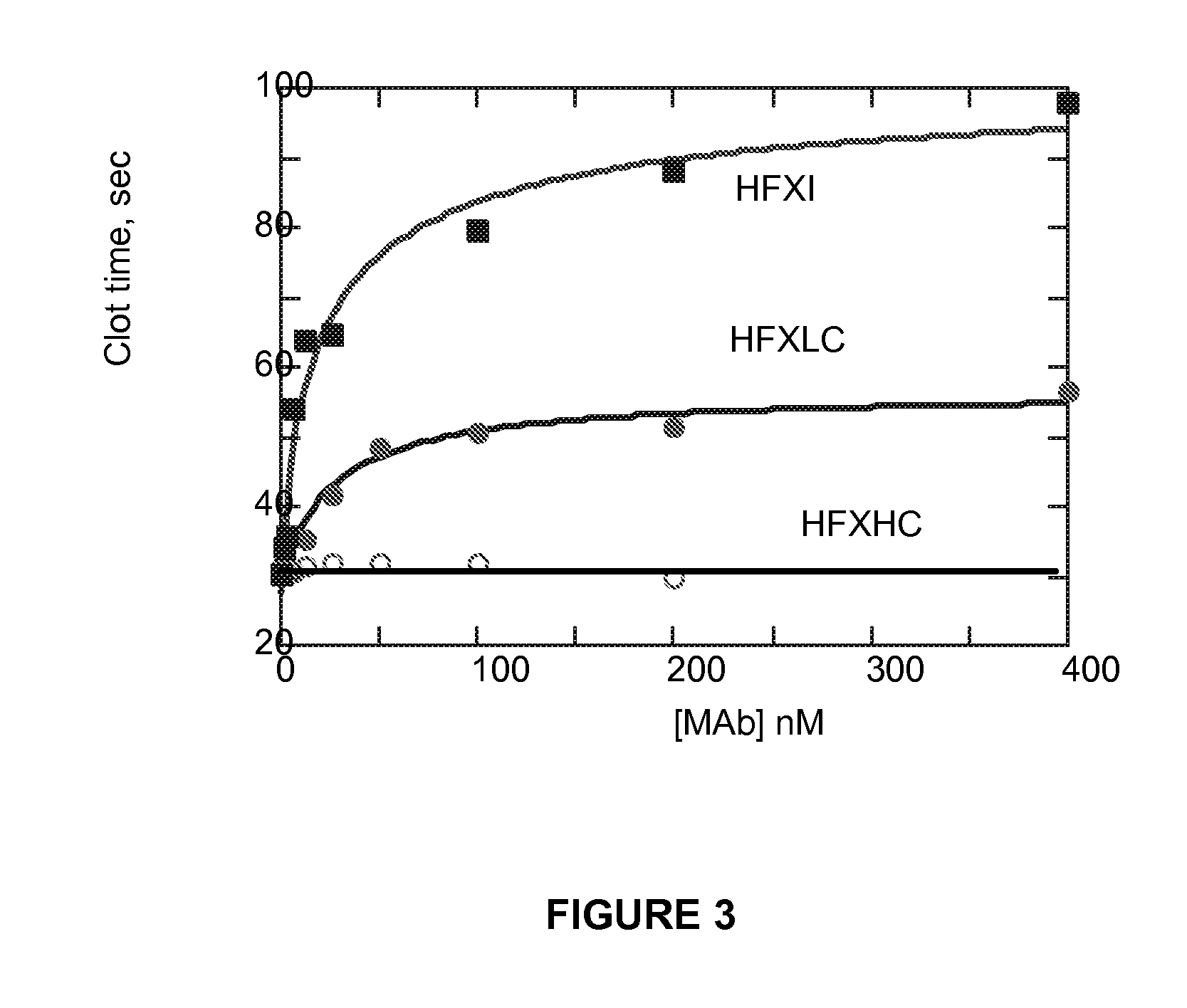
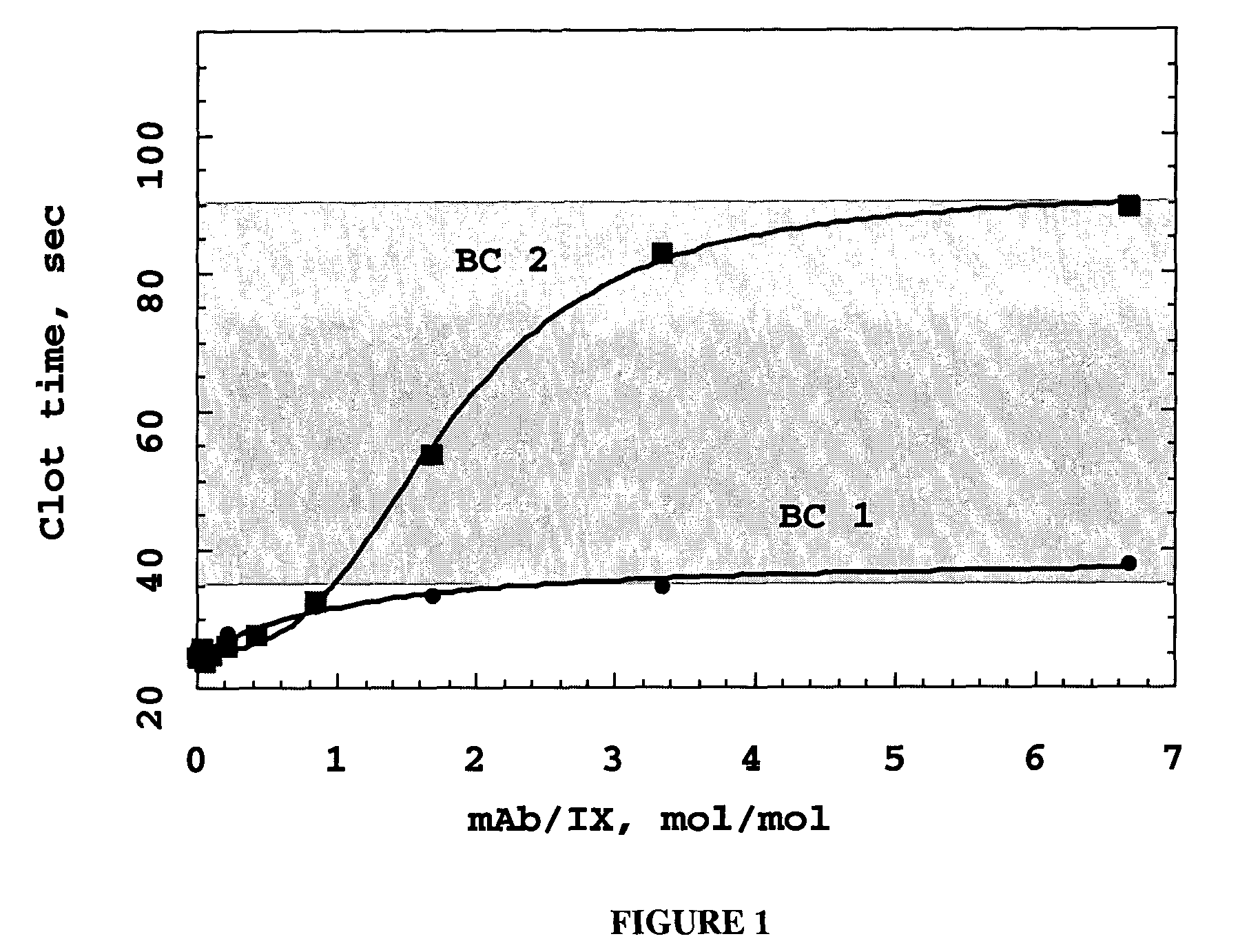
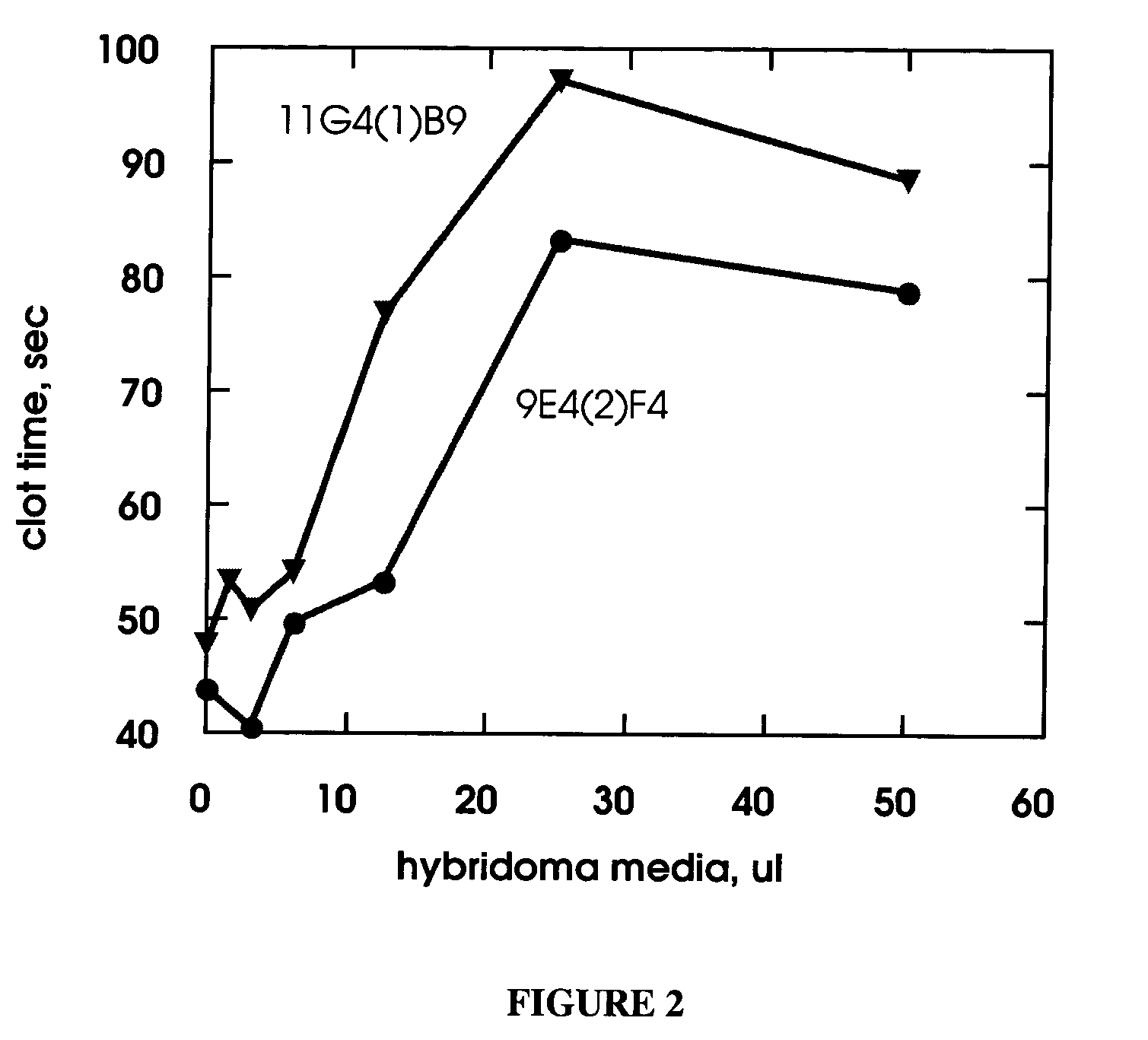
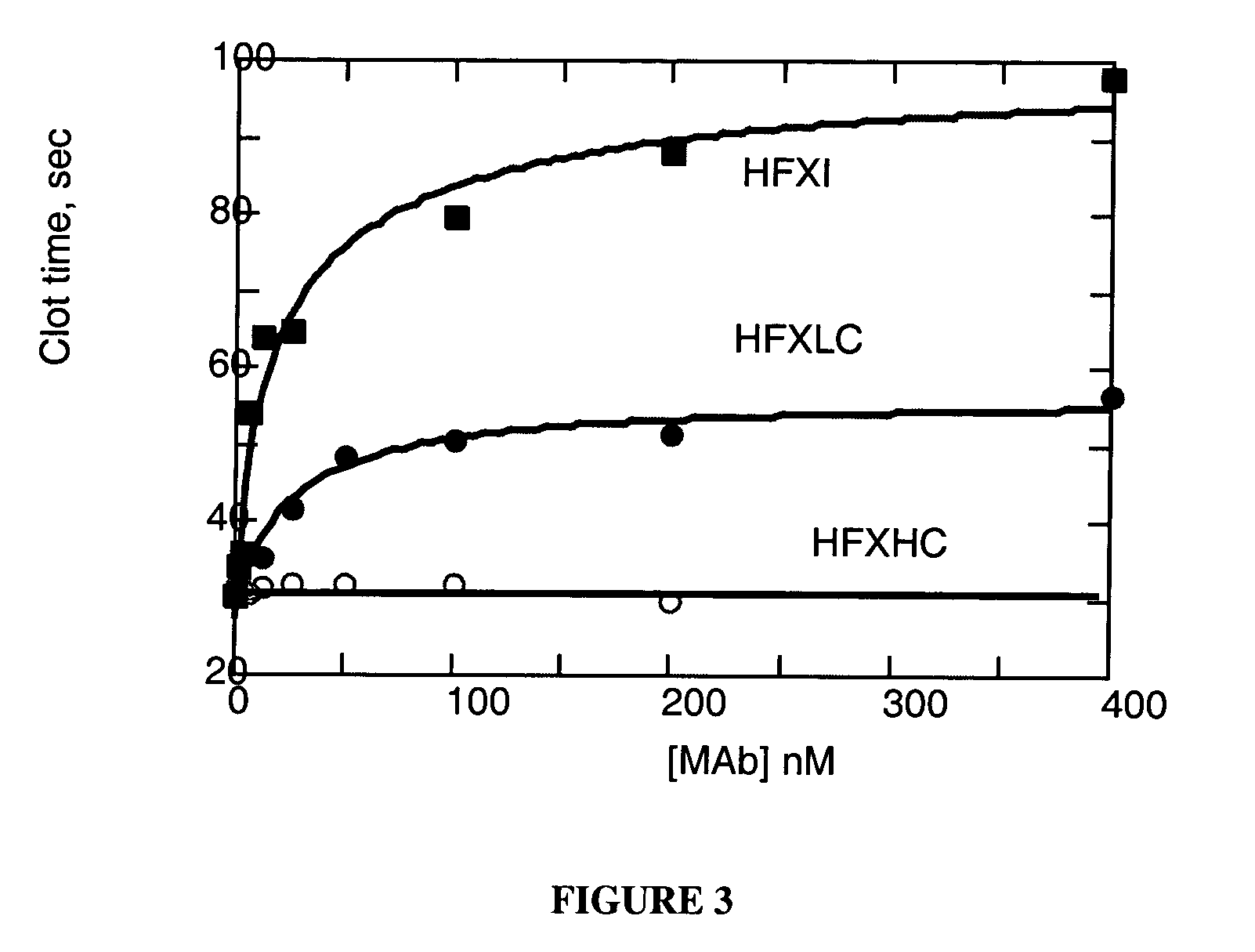
Monoclonal antibodies directed against coagulation factor IX and their use in inhibiting thrombosis are disclosed.
Owner:SMITHKLINE BECKMAN CORP
Antithrombotic agents
InactiveUS20070190059A1Reduce required dosePreventing thromboembolic strokeImmunoglobulins against blood coagulation factorsAntibody ingredientsAntithrombotic AgentMonoclonal antibody
Monoclonal antibodies directed against coagulation factor IX and their use in inhibiting thrombosis are disclosed.
Owner:UNIVERSITY OF VERMONT
Methods for inactivating pathogens using broad-spectrum pulsed light
InactiveCN1344170AImprove methodReliable methodPeptide/protein ingredientsInactivation/attenuationBovine Viral Diarrhea VirusesWhole blood product
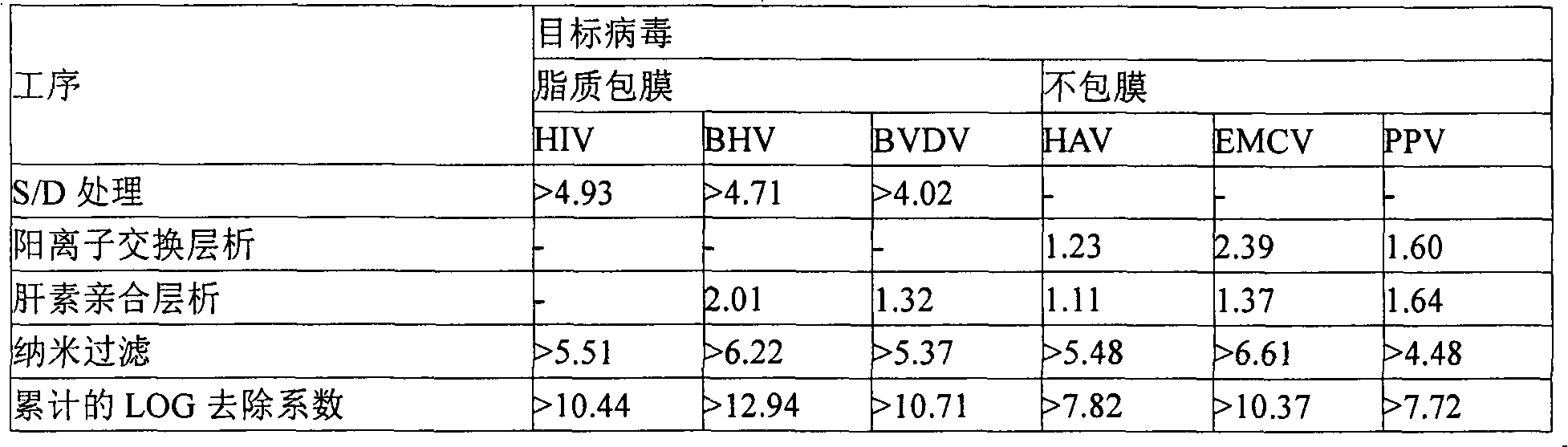
A method of reducing pathogen content in a biologically derived composition by irradiating it with at least one high-intensity short-duration pulse of broad-spectrum incoherent polychromatic light. The biomolecule of interest retains biological activity in the resulting treated composition. Biologically derived compositions, such as serum, plasma or other blood products, including insulin, transferrin, heparin, collagen, coagulation factor VIII and / or coagulation factor IX, or containing monoclonal antibodies, or genetically engineered cell lines, etc. The content of pathogens, such as viruses, bacteria, pyrogens, fungi and / or prions present in the resulting protein composition is reduced by irradiation with broad spectrum pulsed light. A single pulse of broad spectrum light significantly reduces the amount of HIV-1, SV40, canine parvovirus or bovine viral diarrhea virus in a biologically derived composition.
Owner:PUREPULSE TECH
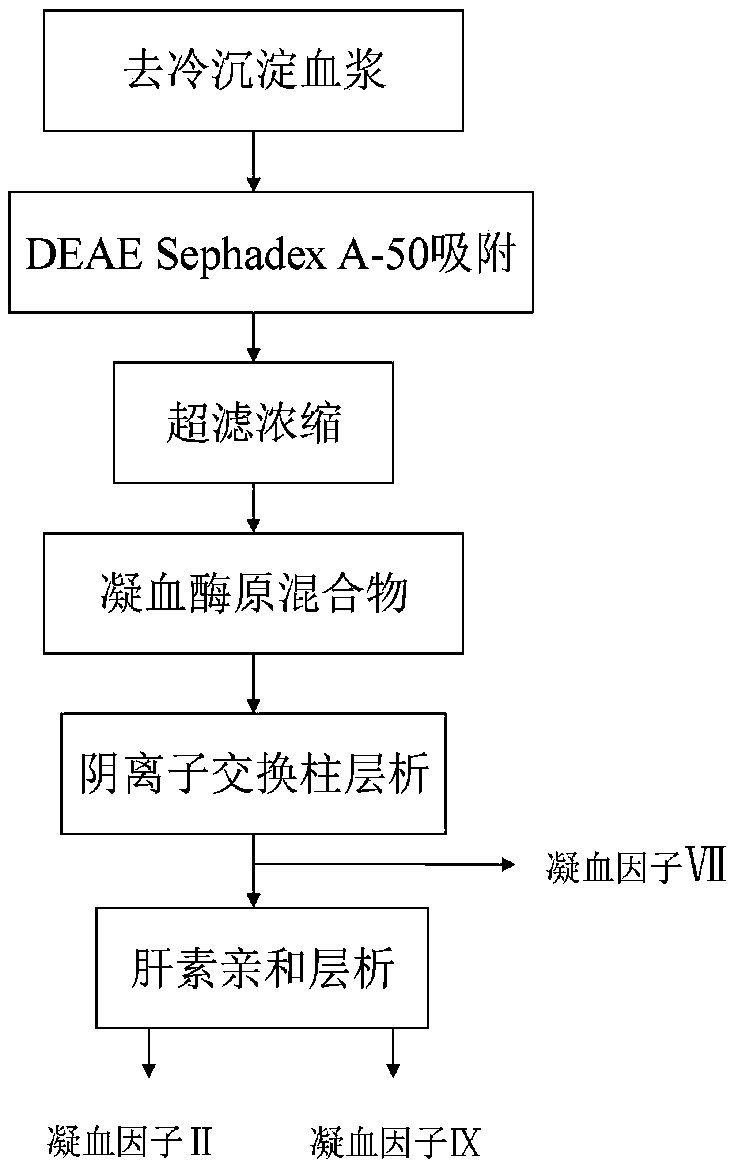
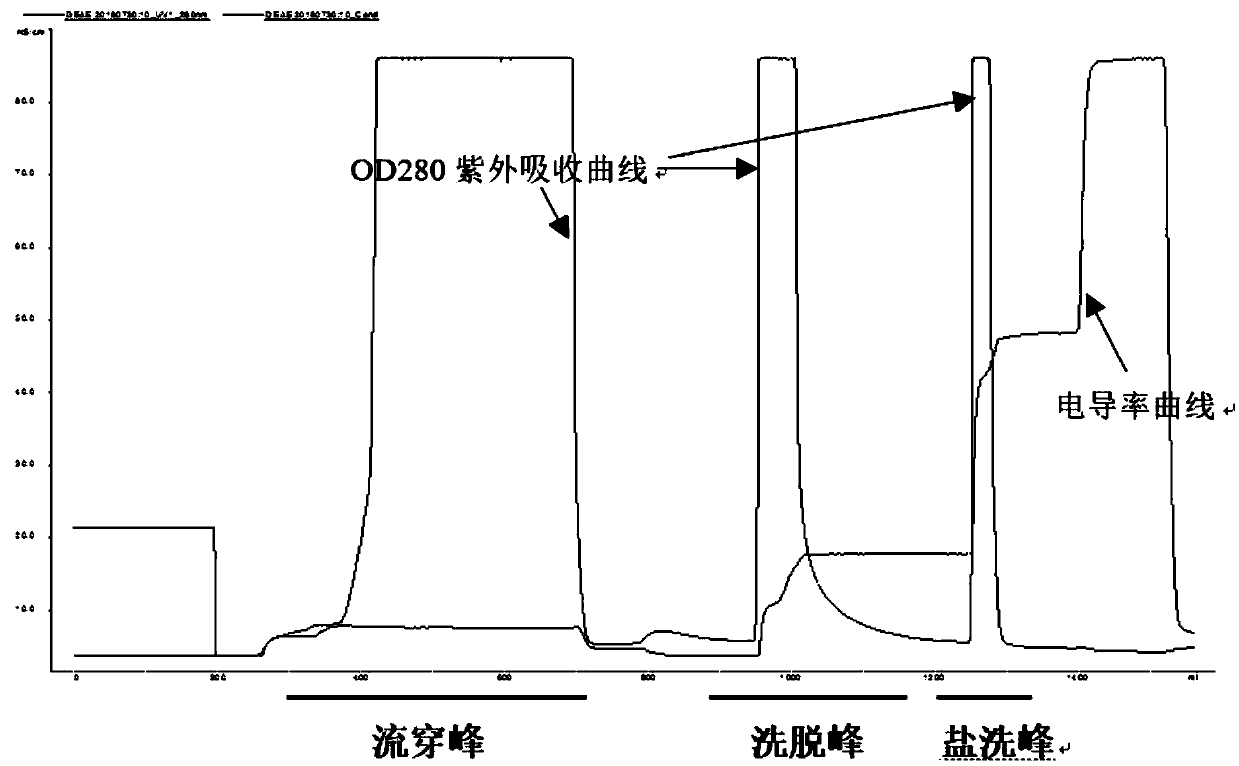
Method for simultaneously separating and purifying coagulation factors IX, X and VII from human plasma
ActiveCN109651502AAchieve separationEasy to operateFactor VIIPeptide preparation methodsUltrafiltrationAnion-exchange chromatography
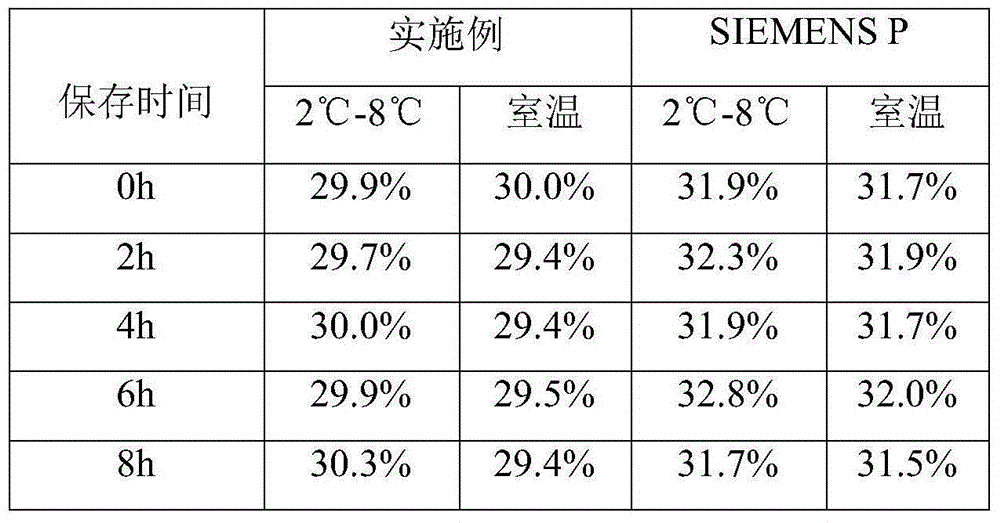
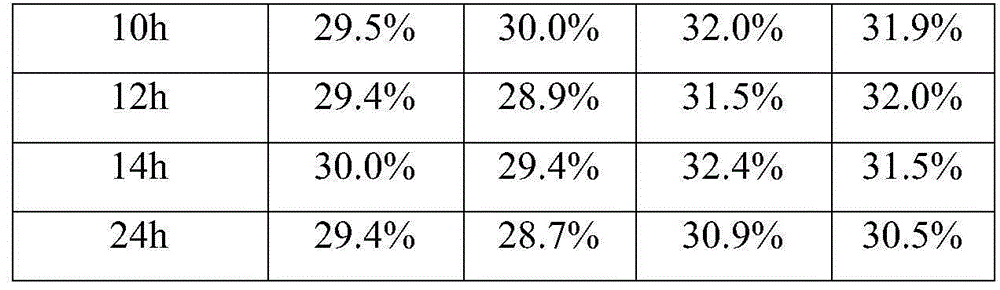
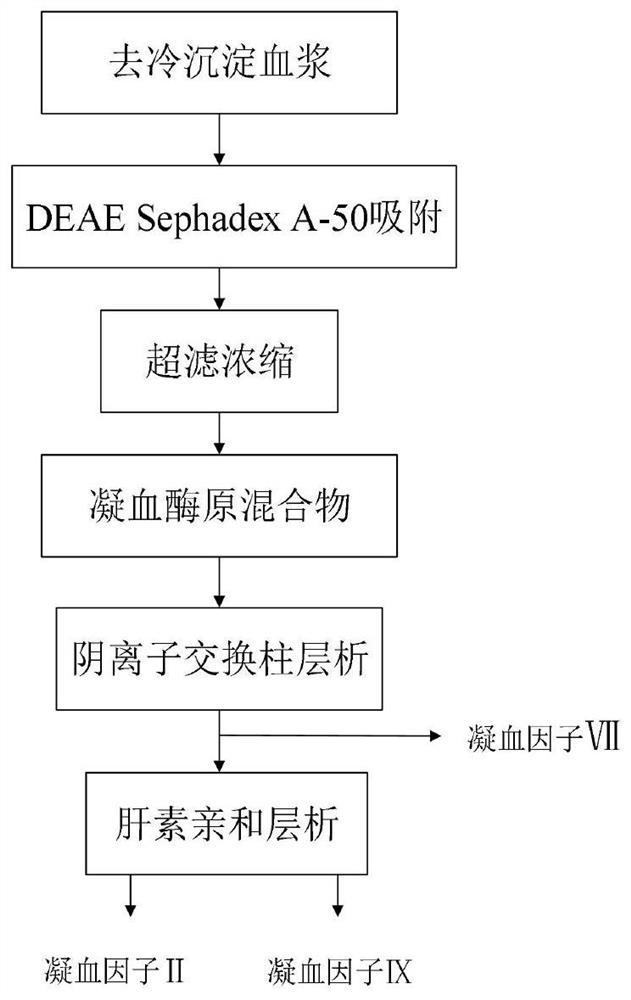
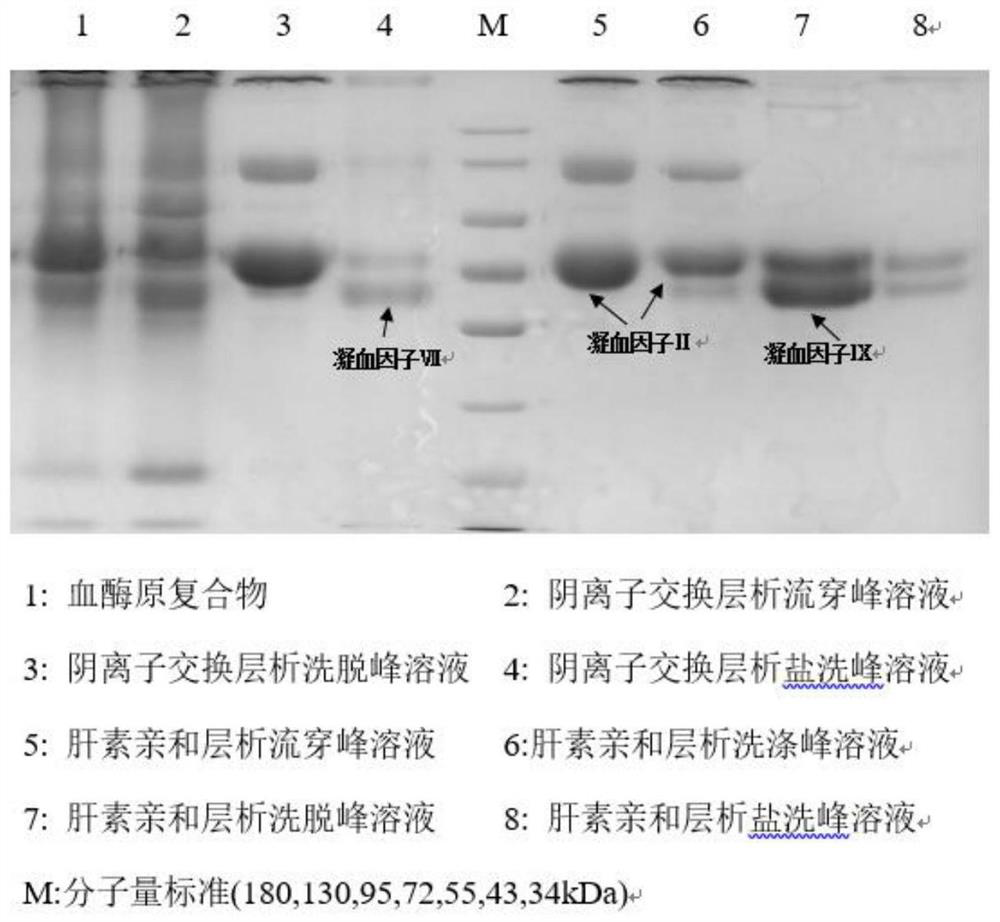
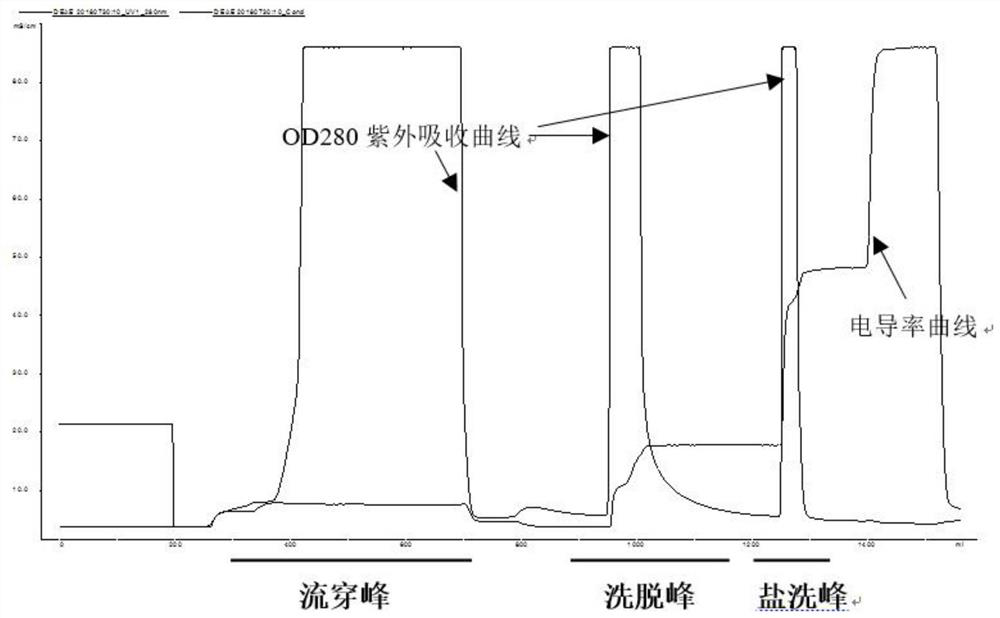
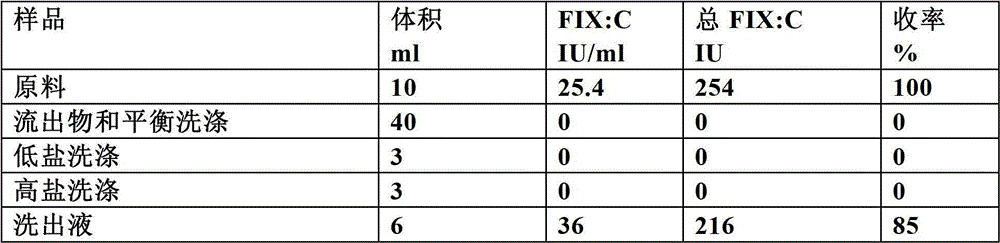
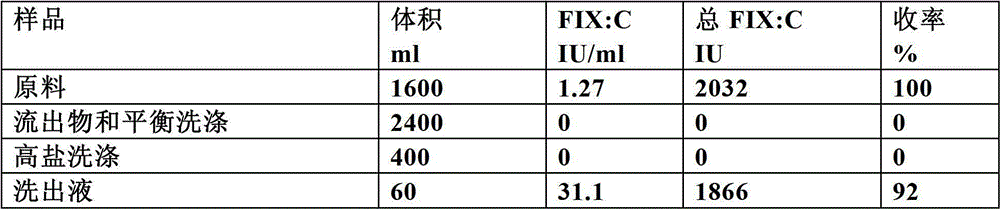
The invention discloses a method for simultaneously separating and purifying coagulation factors IX, X and VII from human plasma, which comprises the following steps: performing centrifugal impurity removal, gel adsorption and ultrafiltration concentration to prepare a prothrombin compound; separating the coagulation factors VII and a mixed solution containing IX and II through an anion exchange resin column; separating the coagulation factor II and IX from the mixed solution containing IX and II by affinity chromatography. According to the method, by combining anion exchange chromatography with heparin affinity chromatography, the separation and preparation of three coagulation factors II, VII and IX at the same time can be achieved; the method has the advantages of high raw material utilization, simple operation and short time consumption; meanwhile, by detecting the electric signals in the chromatography process, the corresponding coagulation factors are accurately collected, the purity of the coagulation factors is effectively improved, and the economic benefit is improved.
Owner:HUALAN BIOLOGICAL ENG INC +2
Antithrombotic agents
InactiveUS20050037006A1Reduce required dosePreventing thromboembolic strokeImmunoglobulins against blood coagulation factorsPeptide/protein ingredientsAntithrombotic AgentMonoclonal antibody
Monoclonal antibodies directed against coagulation factor IX and their use in inhibiting thrombosis are disclosed.
Owner:UNIVERSITY OF VERMONT
Pharmaceutical composition of hemocoagulase and application of pharmaceutical composition
PendingCN108785665APromote aggregationAggregate recoveryPeptide/protein ingredientsPharmaceutical delivery mechanismFIBRINOGEN/THROMBINHaemophilia B
The invention discloses a pharmaceutical composition of hemocoagulase. The pharmaceutical composition is characterized in that the pharmaceutical composition is prepared from 0.00005 to 0.0005 percentof a hemocoagulase composition and the balance of a pharmaceutically acceptable carrier. The composition is applied to preparation of a medicine for treating Haemophilia B (coagulation factor IX deficiency). Active components in the hemocoagulase comprise hemocoagulase, i.e., a thrombin-like enzyme (batroxobin), and a phospholipid dependent coagulation factor X activating agent (FXA). A bleedingstopping process of the hemocoagulase does not depend on a coagulation factor IX and is mainly used for fibrin and an activated coagulation factor X, and has the effect of treating bleeding of the haemophilia B. After the hemocoagulase is used for treating, platelet aggregation can be remarkably enhanced, and the formation of blood clots or fibrin clots in a patient with the haemophilia B is recovered.
Owner:ZHAOKE PHARMA HEFEI
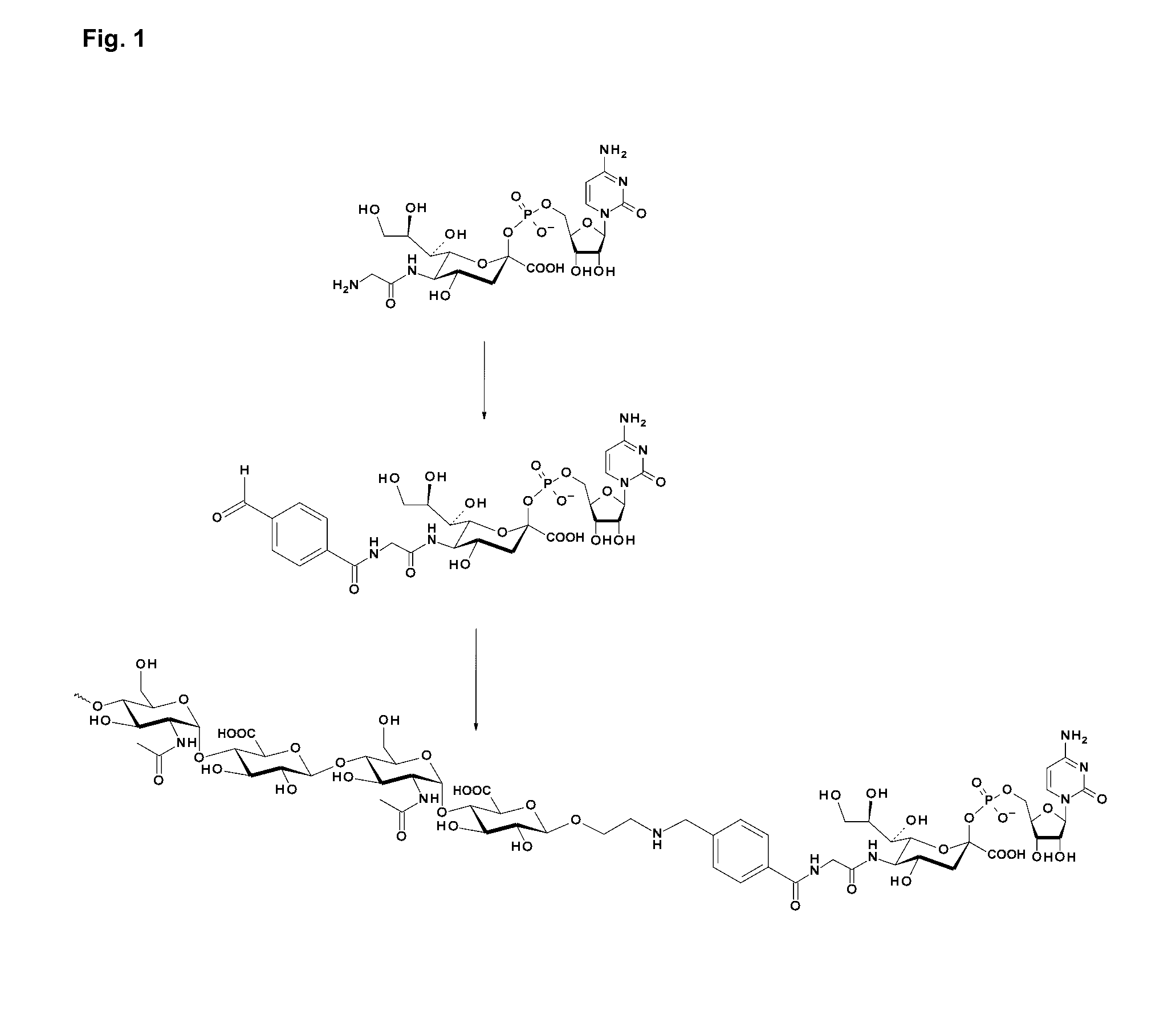
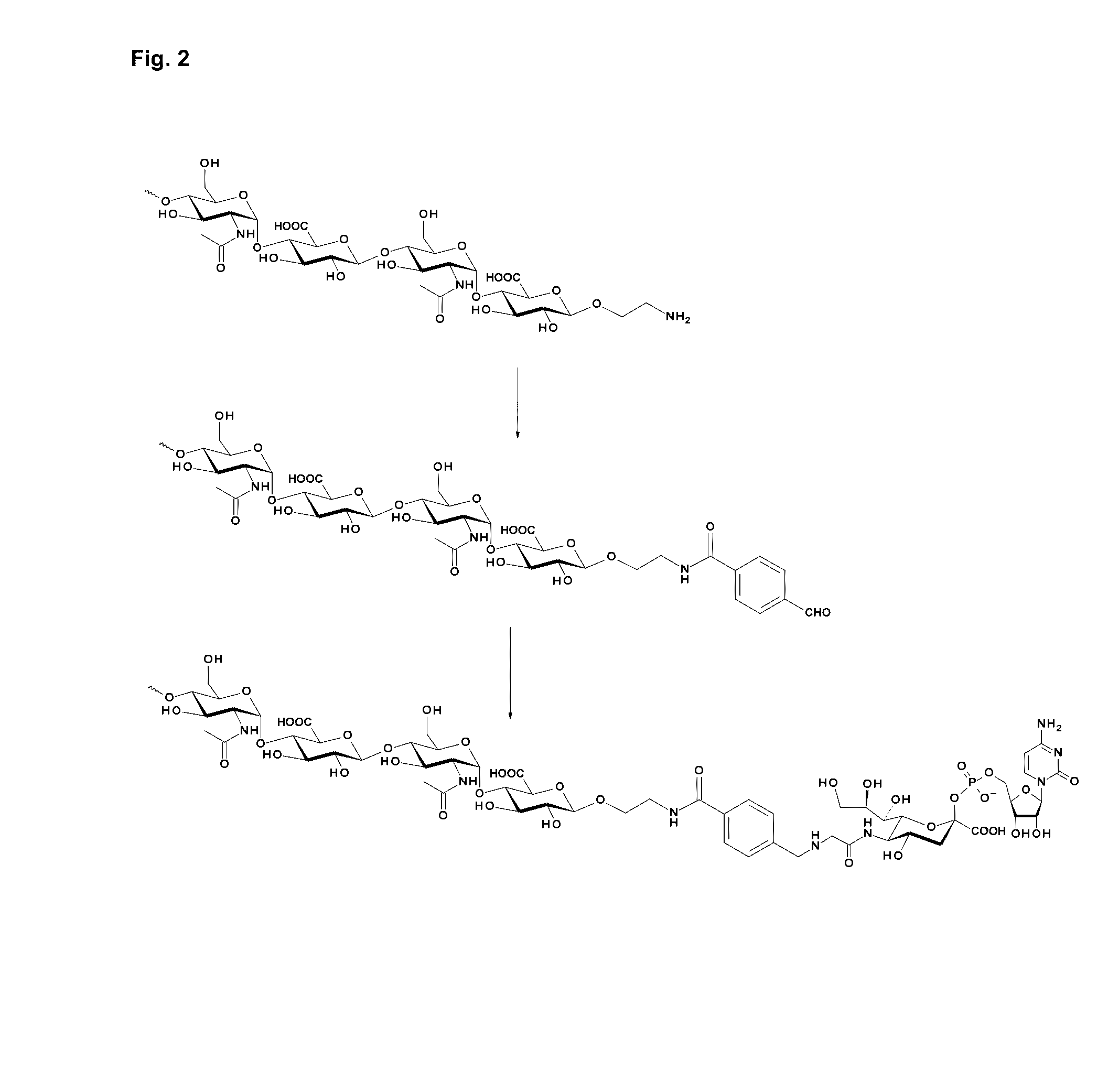
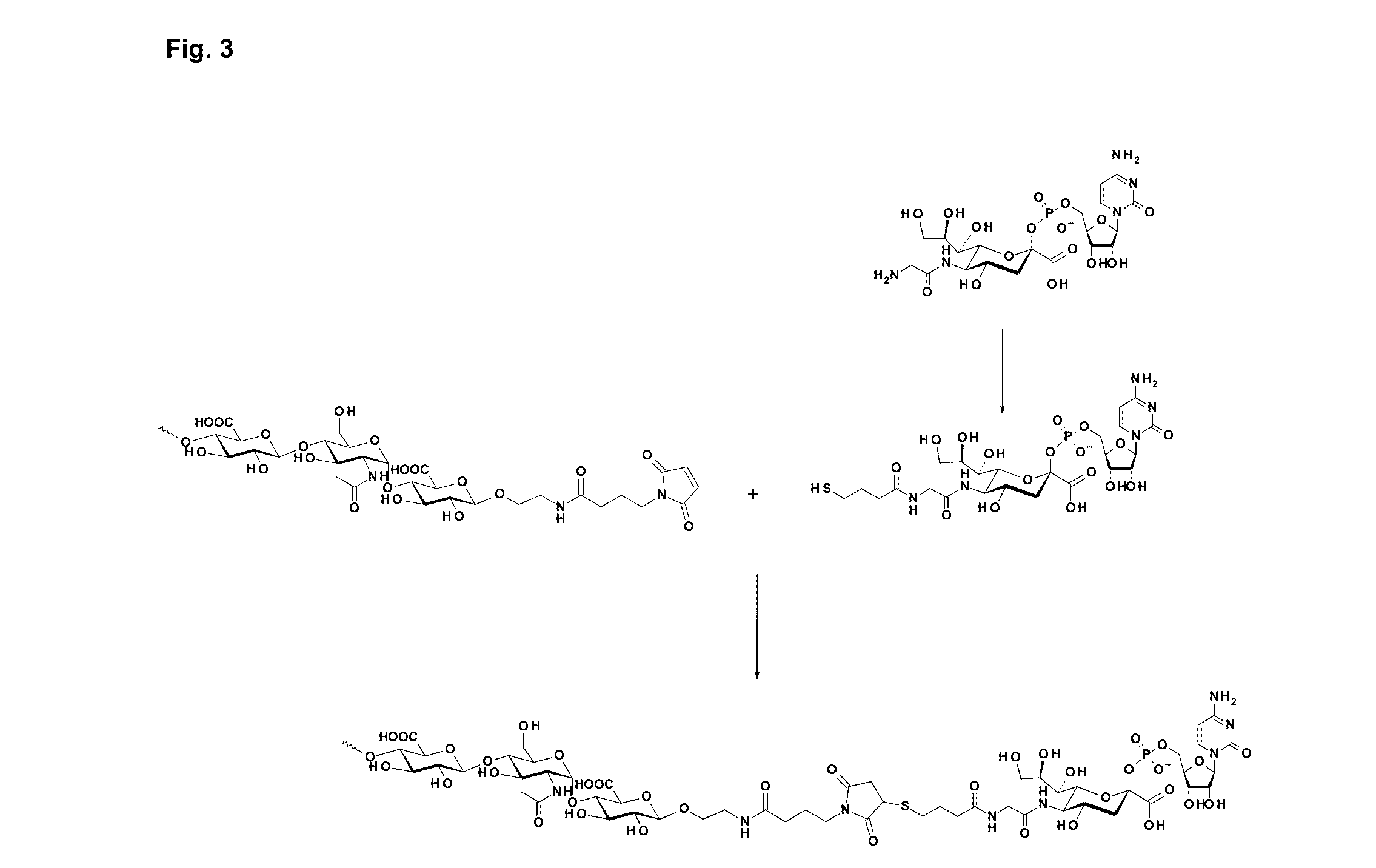
Coagulation factor IX conjugates
The present invention relates to Factor IX polypeptides conjugated to heparosan (HEP) polymers, methods for the manufacture thereof and uses of such conjugates. The resultant conjugates may be used - for example - in the treatment or prevention of bleeding disorders such as haemophilia B.
Owner:NOVO NORDISK HEALTH CARE AG
Recombinant adeno-associated virus vectors and methods for treating or preventing hemophilia B
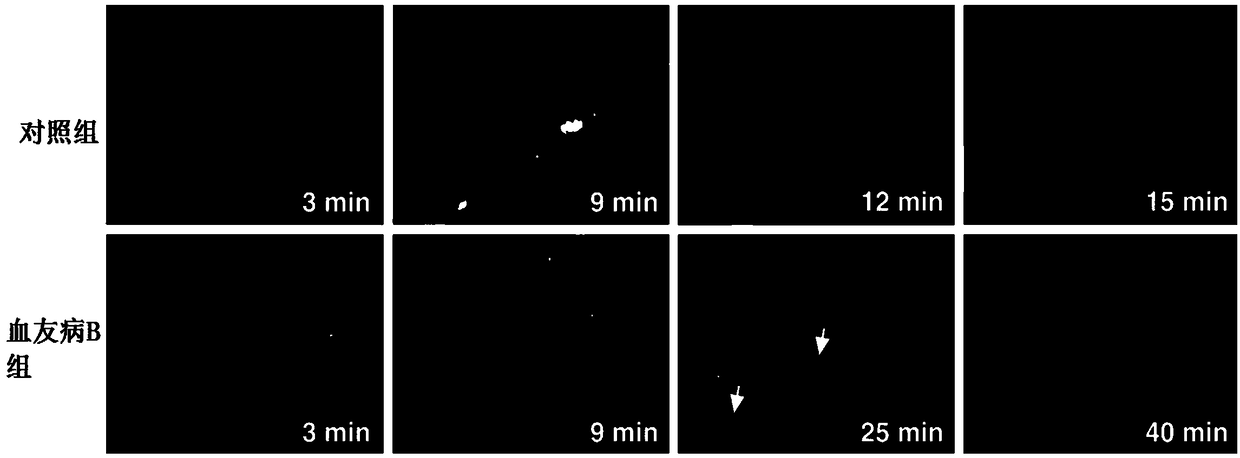
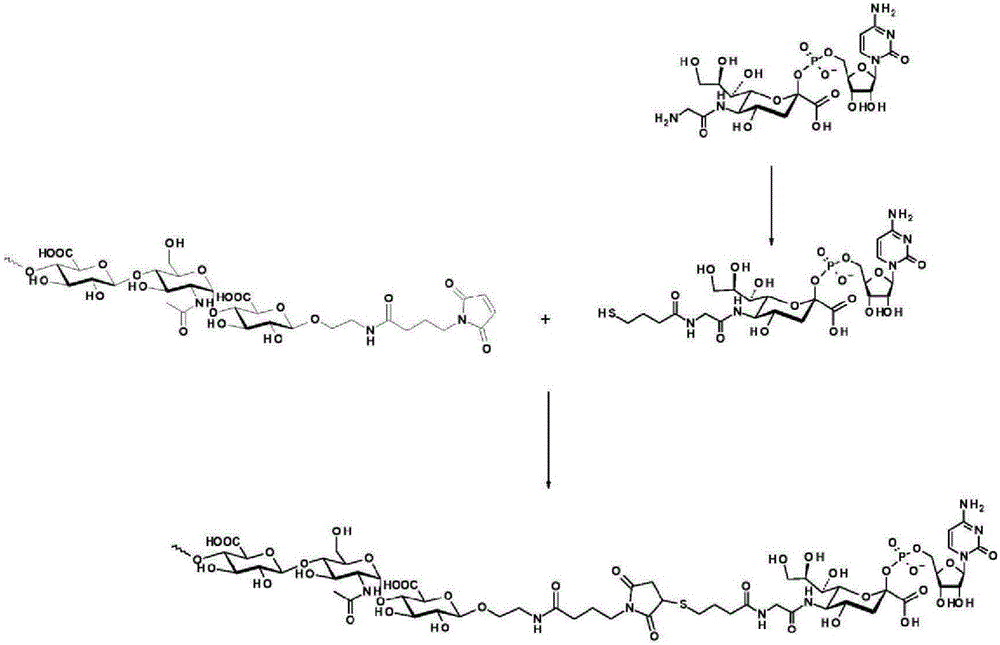
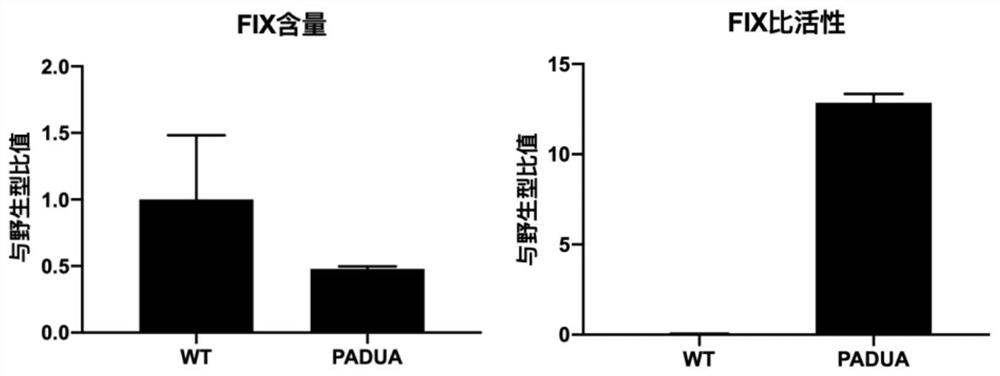
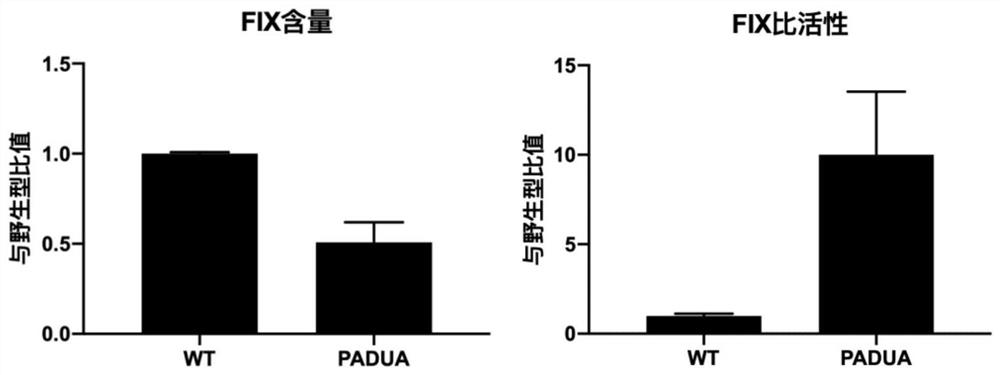
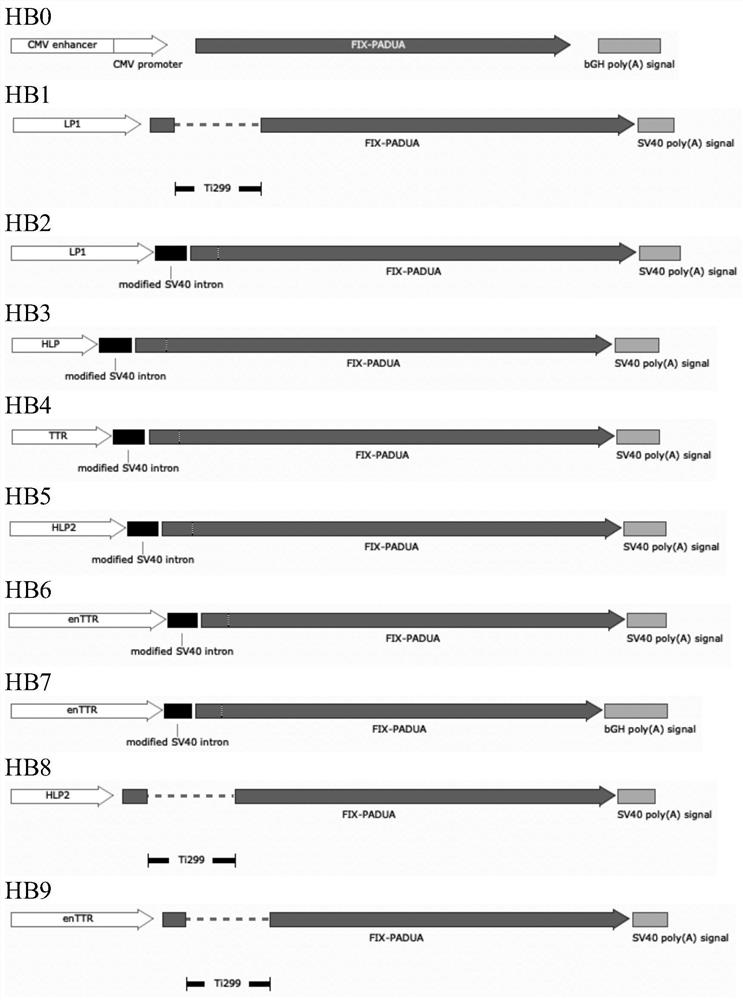
The present invention relates to expression constructs and recombinant adeno-associated virus (rAAV) vectors that express coagulation factor IX, viral particles and pharmaceutical compositions comprising said rAAV vectors, and their use for the treatment or prevention of hemophilia B.
Owner:SHANGHAI VITALGEN BIOPHARMA CO LTD
Single-domain antibody specific to coagulation factor IX (FIX)
ActiveCN110343181AImmunoglobulins against blood coagulation factorsPreparing sample for investigationSingle-domain antibodyMedical biology
The invention relates to the field of medical biology, discloses a single-domain antibody specific to a coagulation factor IX (FIX), particularly discloses a FIX binding molecule derived from the single-domain antibody, and especially discloses application in FIX detection and FIX-poor plasma preparation.
Owner:SUZHOU ALPHAMAB
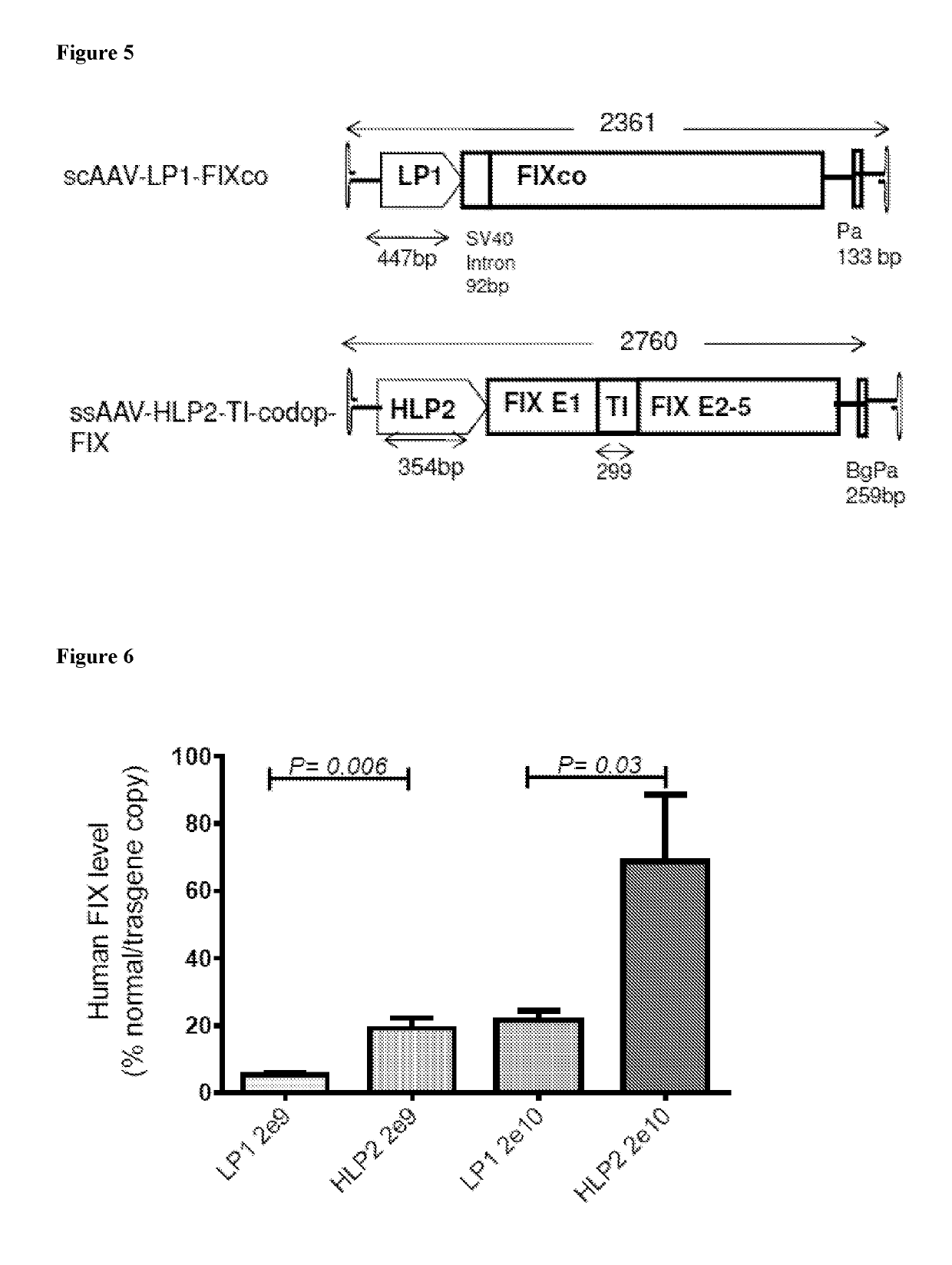
Factor IX gene therapy
ActiveUS10413598B2Improving potency and transduction efficiencyImprove securitySugar derivativesPeptide/protein ingredientsNucleotideIntein
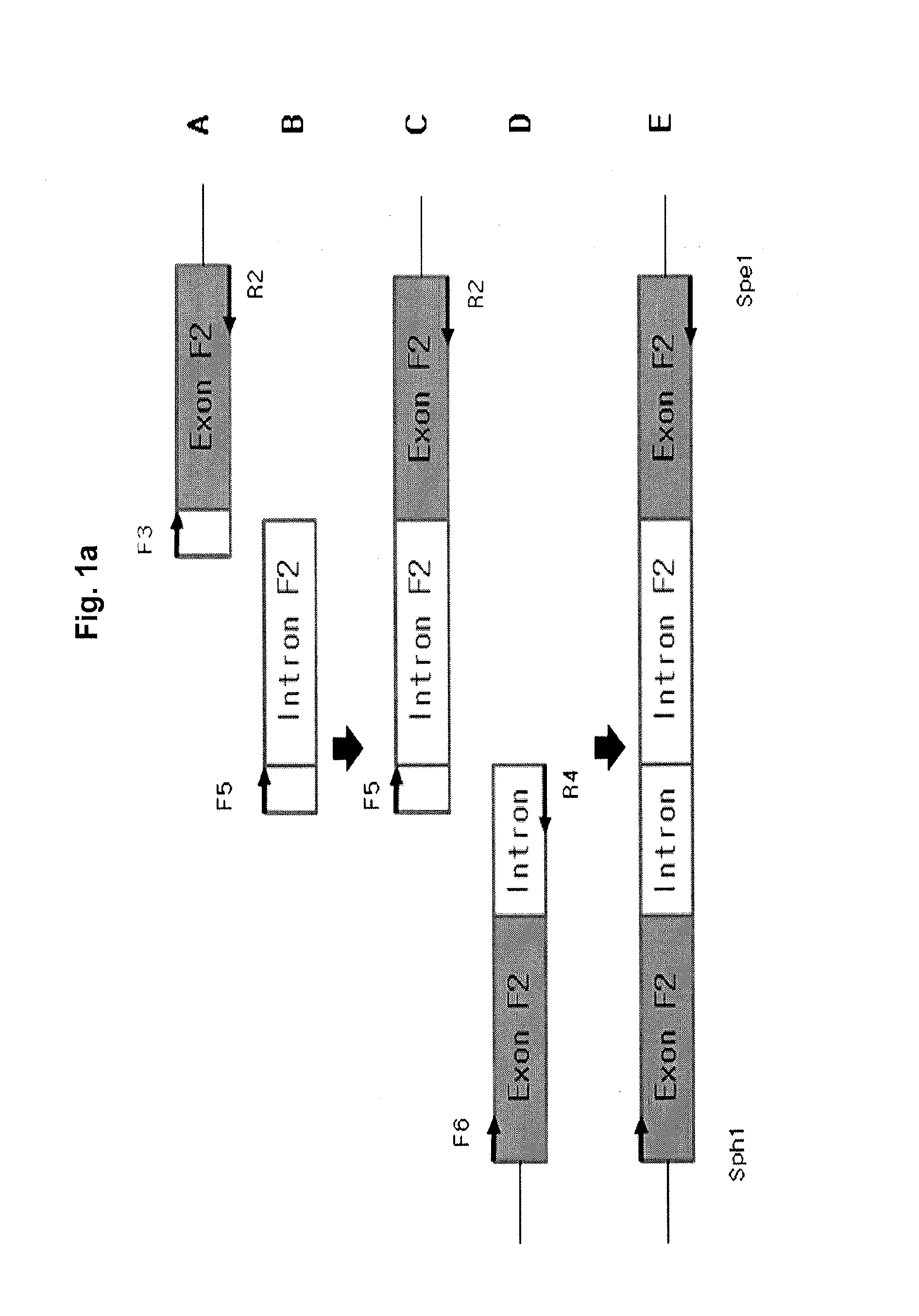
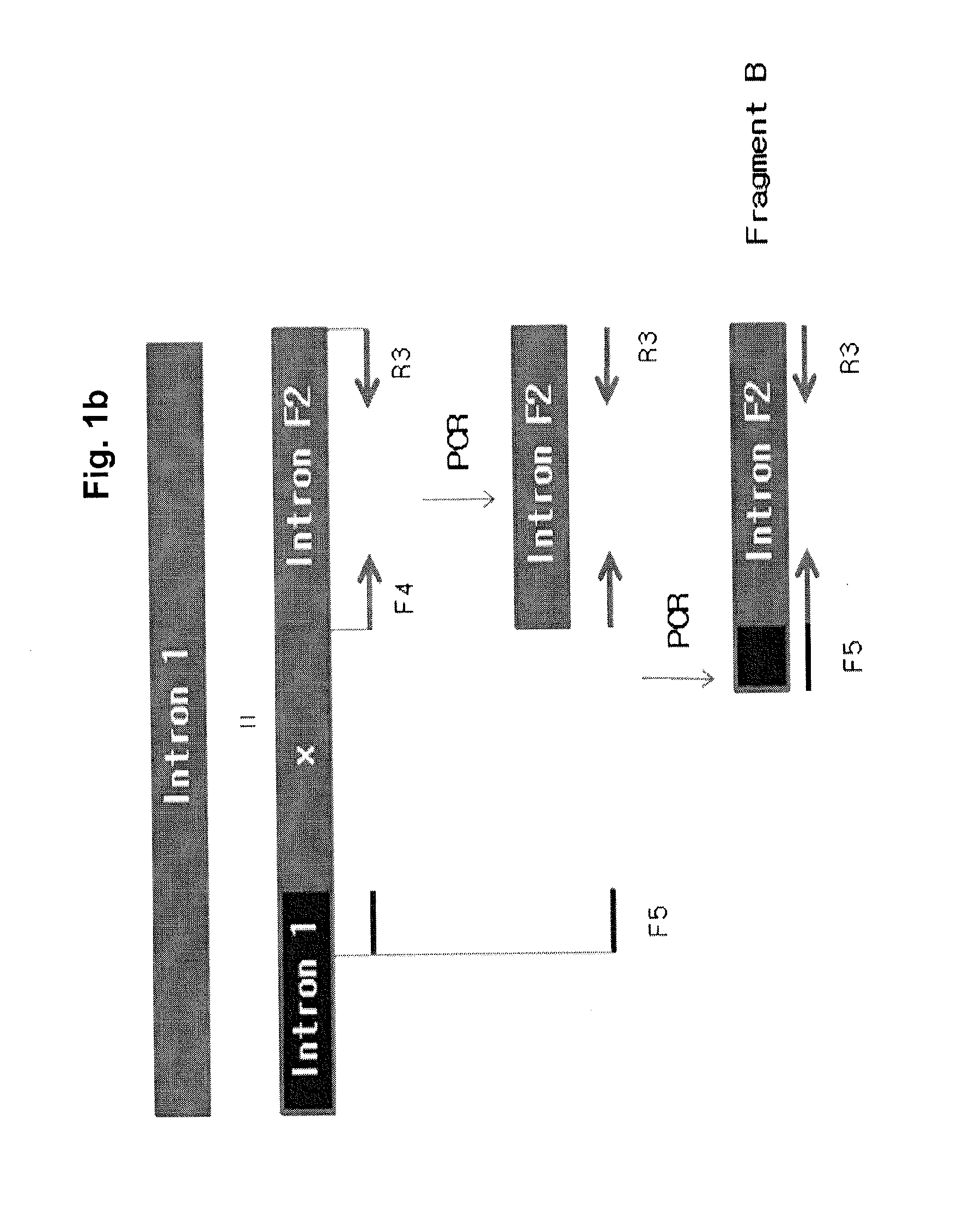
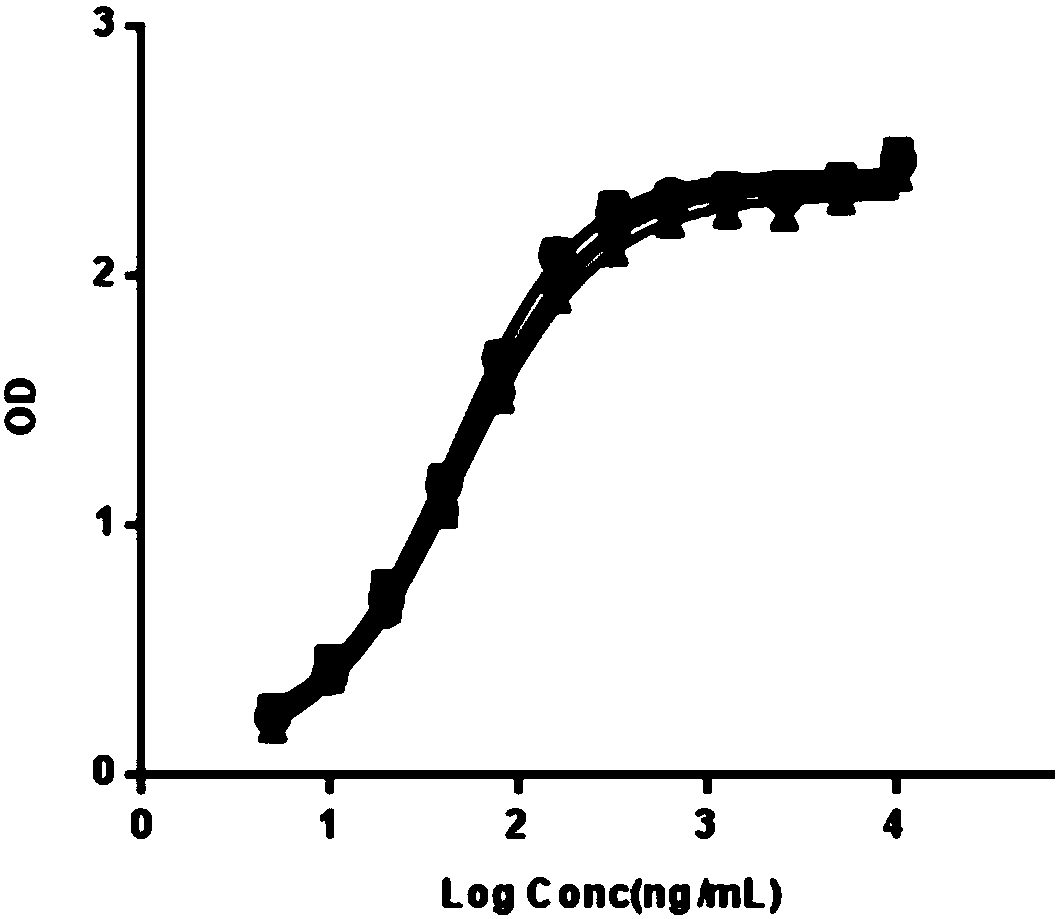
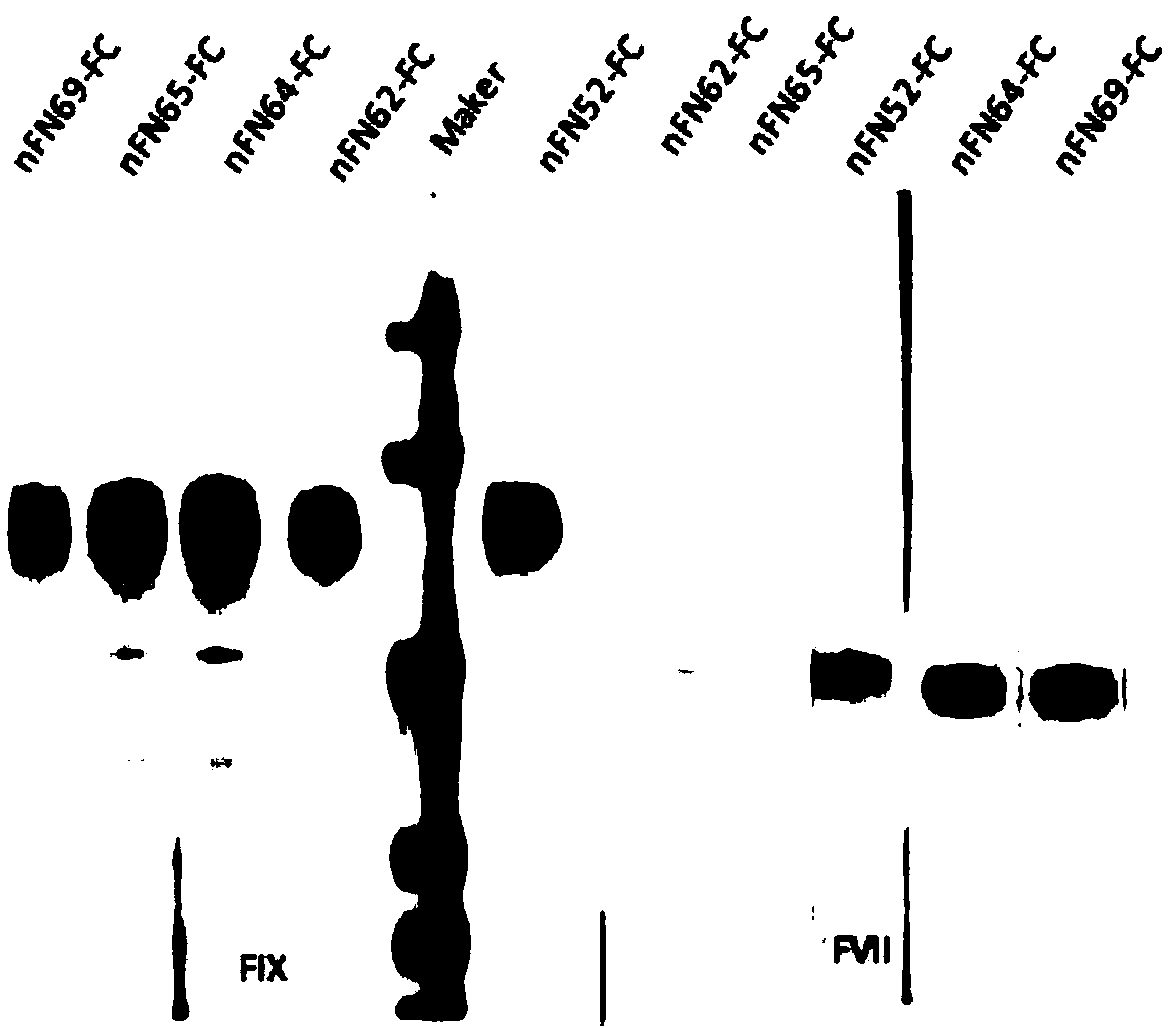
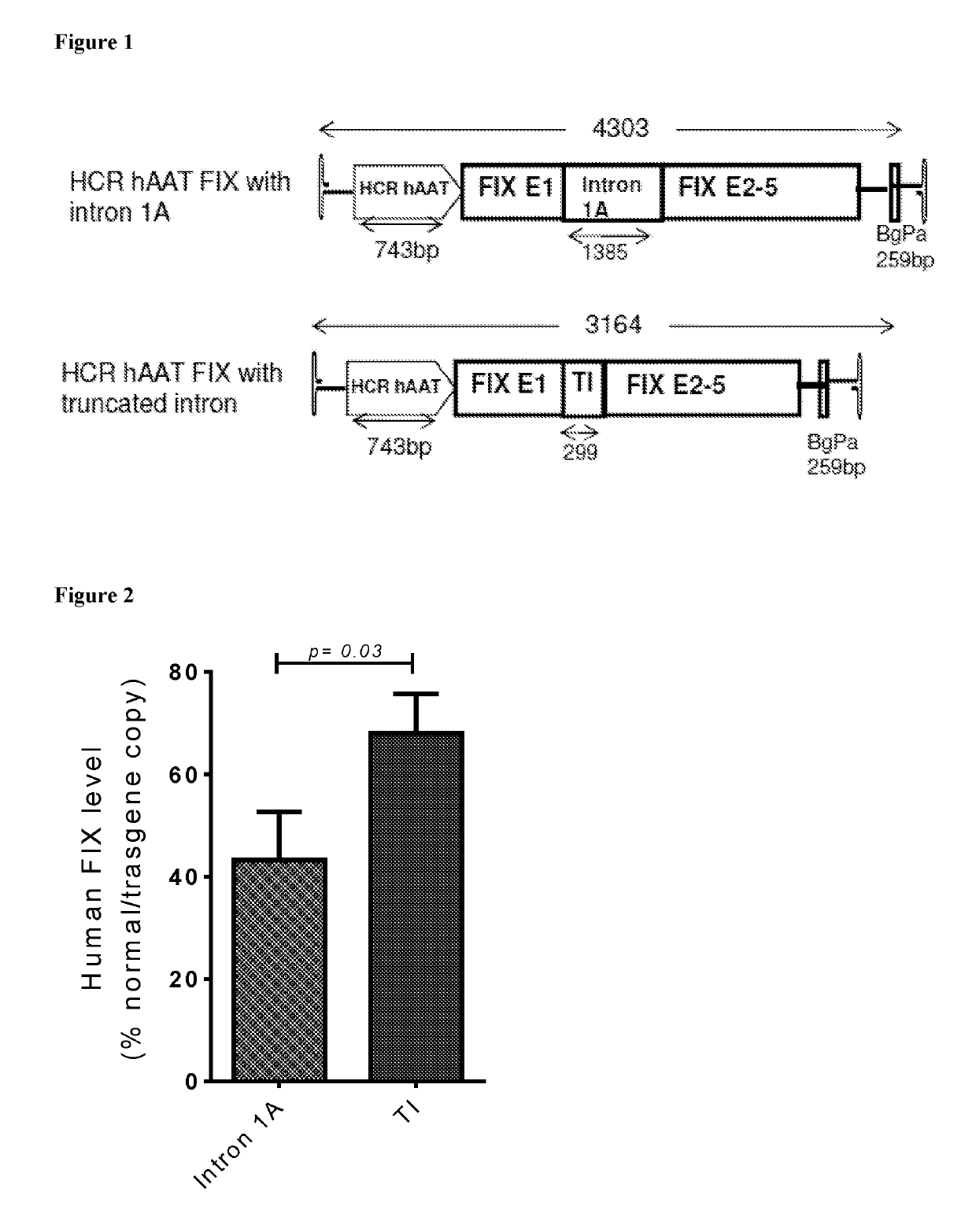
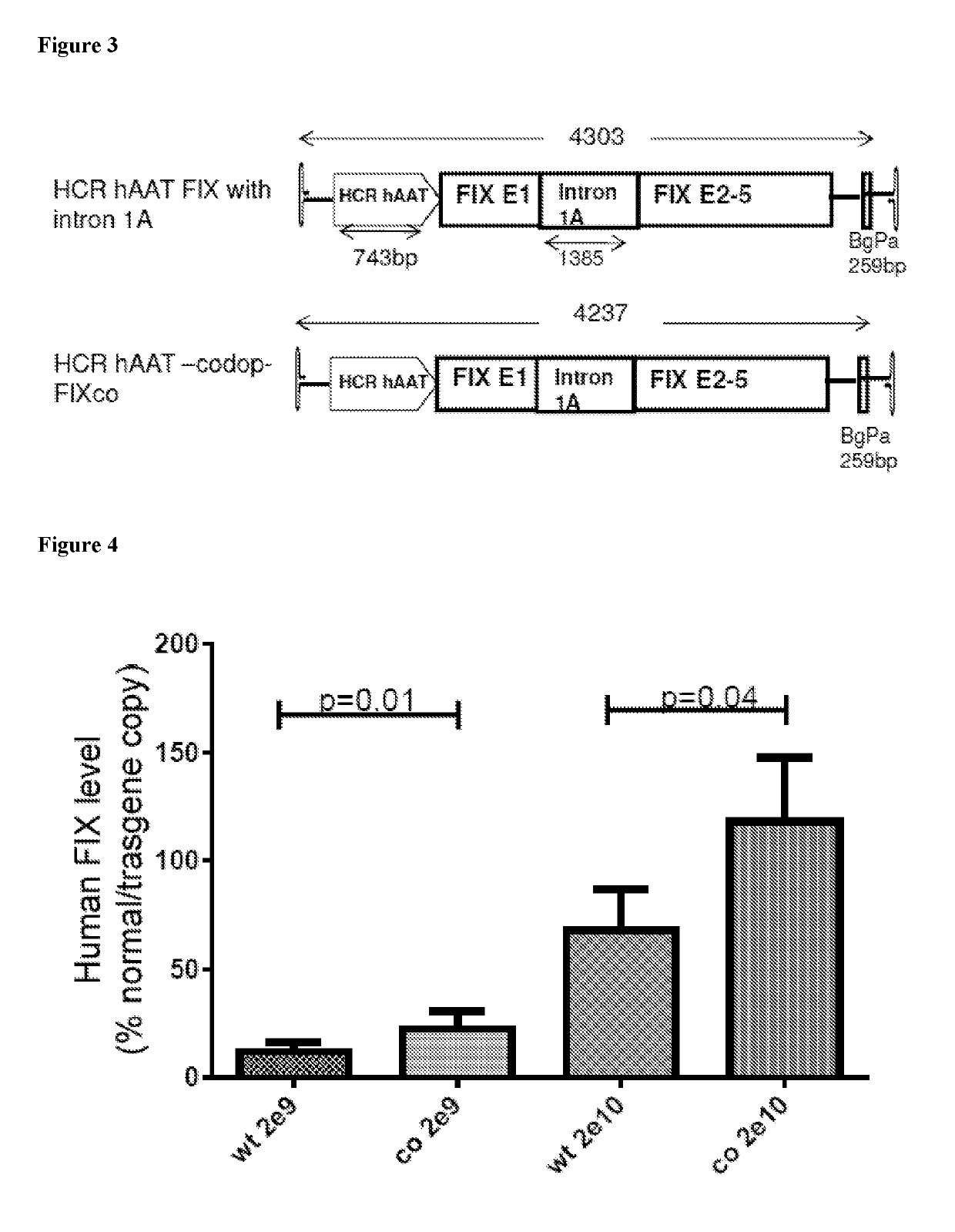
The invention relates to a new, more potent, coagulation factor IX (FIX) expression cassette for gene therapy of haemophilia B (HB). Disclosed is a vector for expressing factor IX protein, the vector comprising a promoter, a nucleotide sequence encoding for a functional factor IX protein and an intron sequence, wherein the intron sequence is positioned between exon 1 and exon 2 of the nucleotide sequence encoding for a functional factor IX protein, and wherein the intron sequence has at least 80% identity to the sequence of SEQ ID NO. 1 as disclosed herein.
Owner:UCL BUSINESS PLC
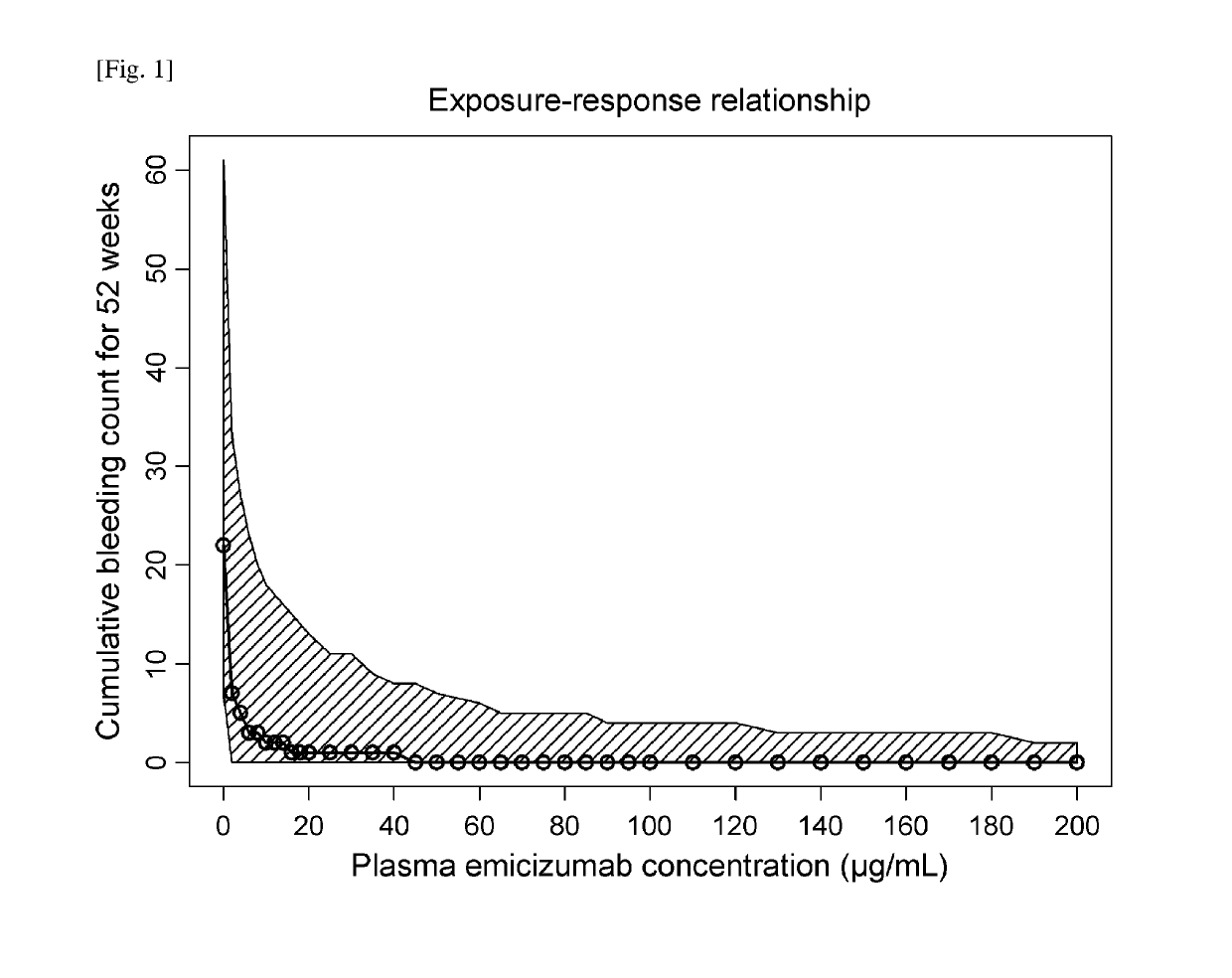
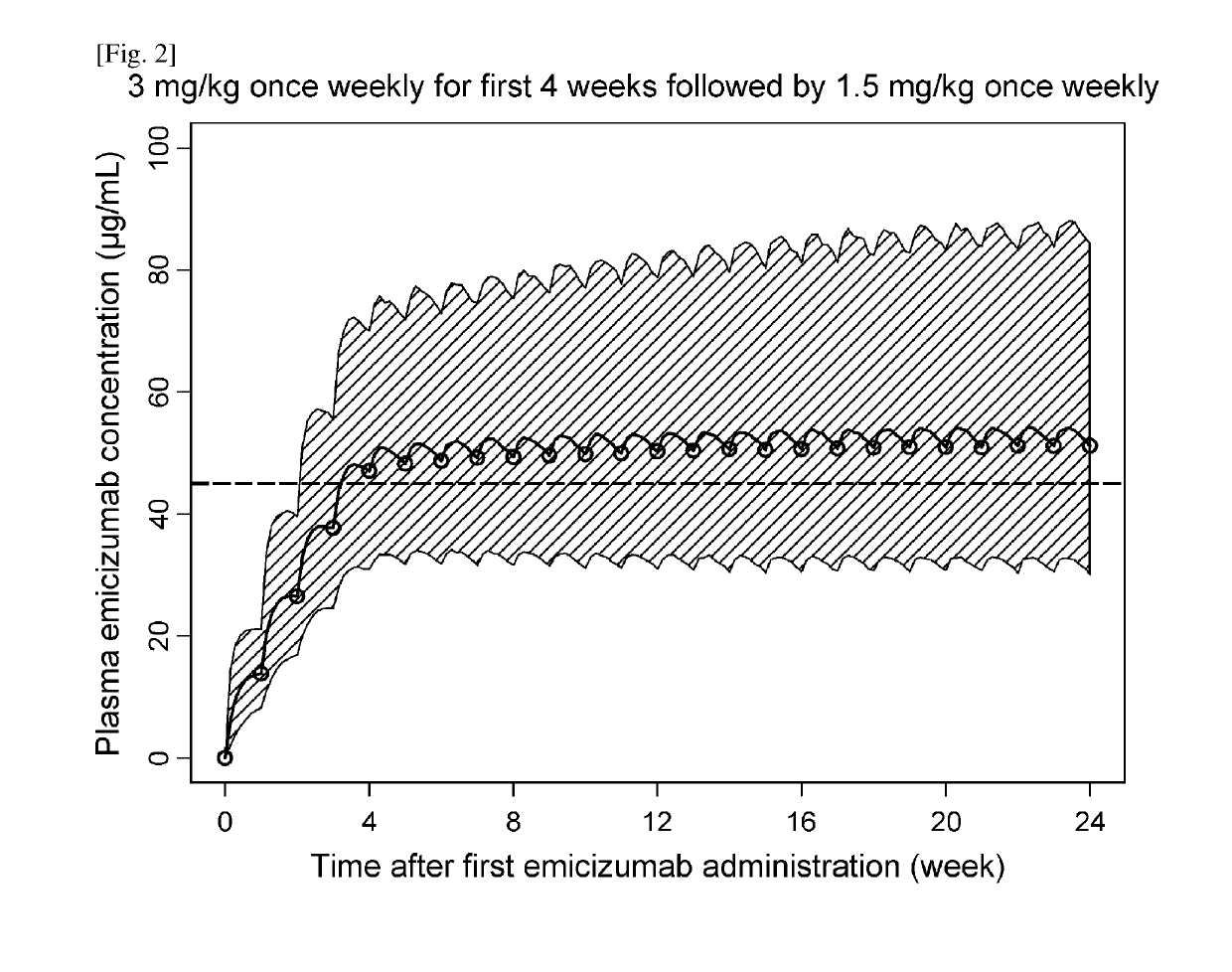
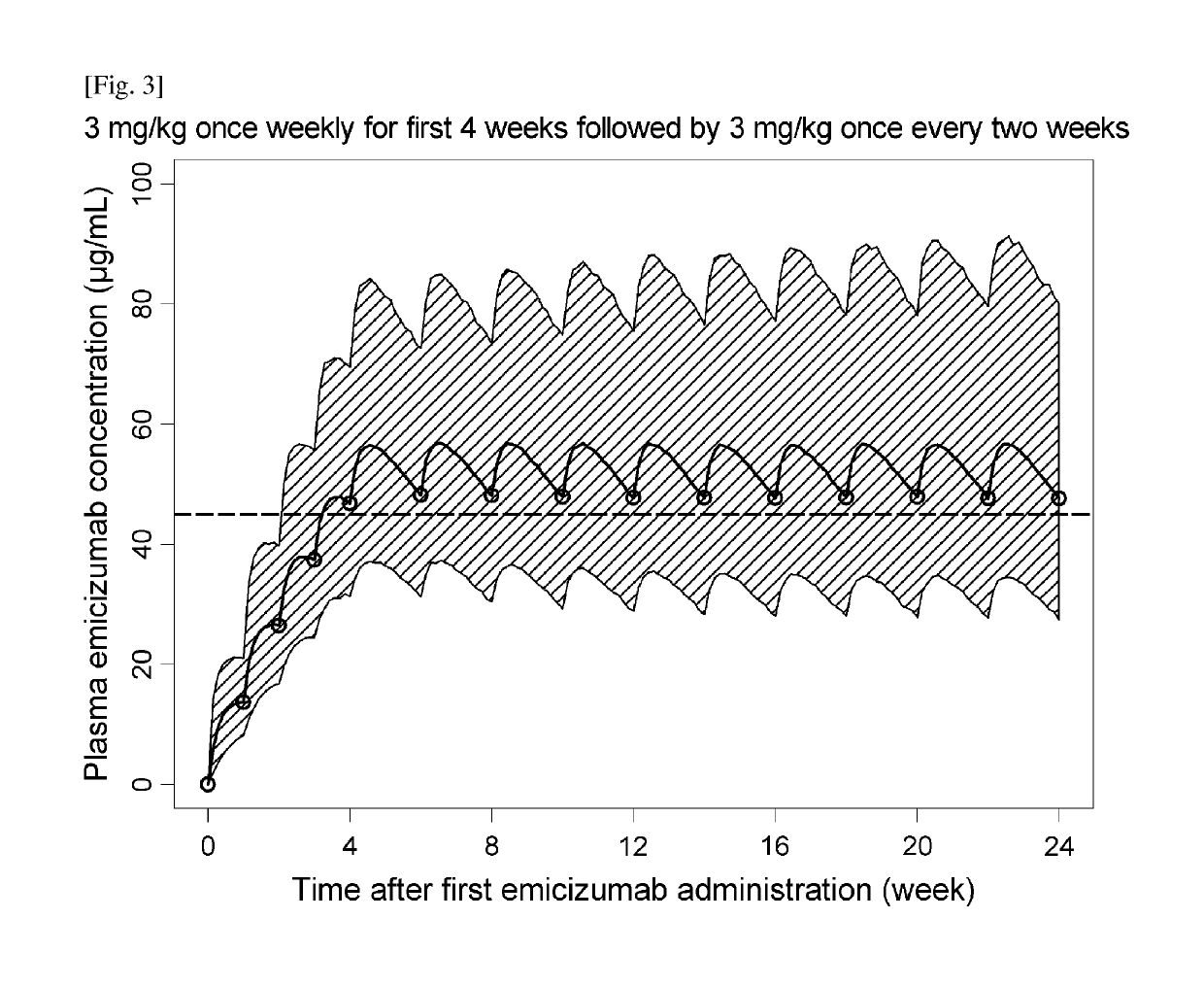
Methods of using a bispecific antibody that recognizes coagulation factor ix and/or activated coagulation factor ix and coagulation factor x and/or activated coagulation factor x
ActiveUS20190194352A1Reduce morbidityImmunoglobulins against blood coagulation factorsAntibody ingredientsRegimenBlood Coagulation Factor X
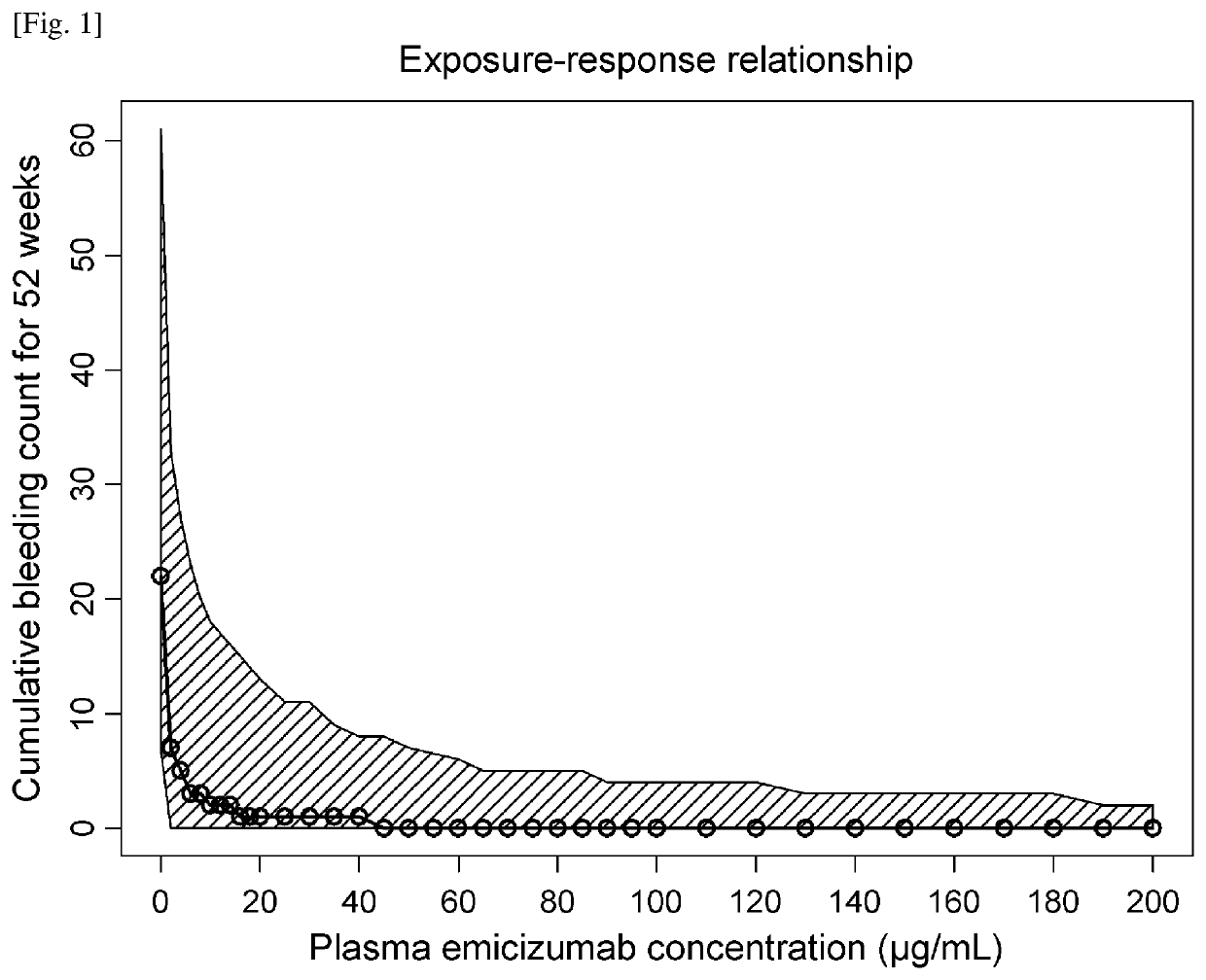
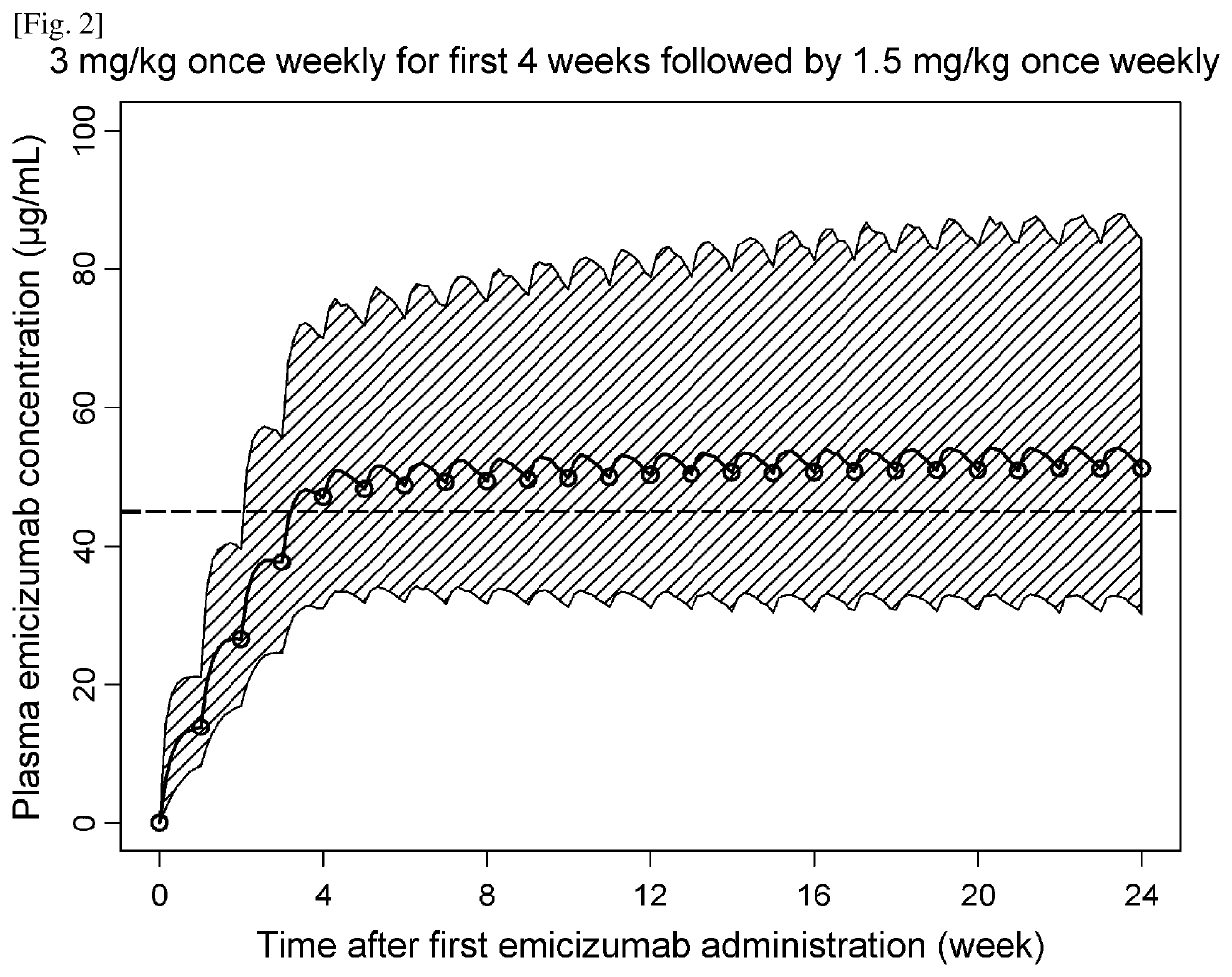
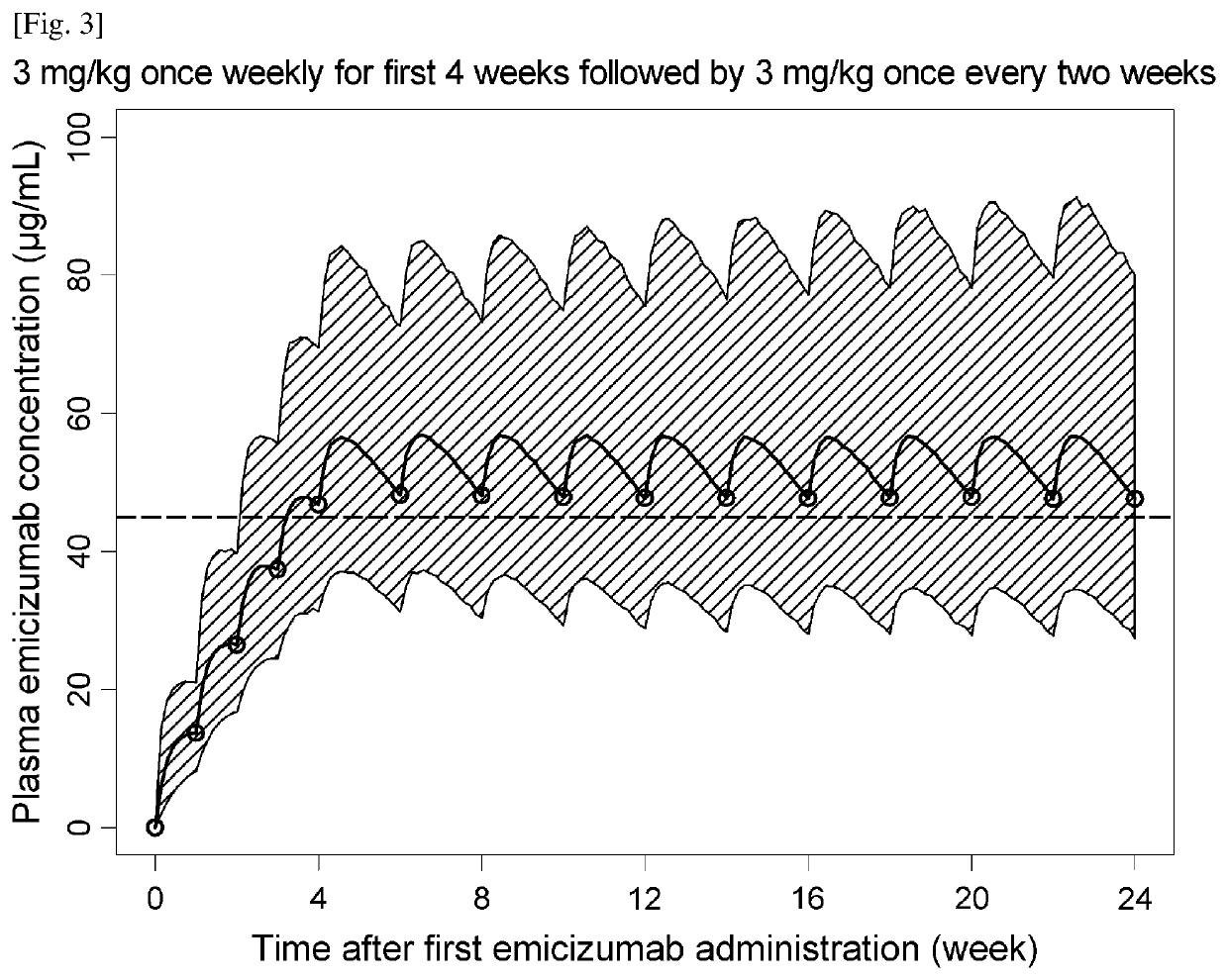
An objective of the present invention is to provide an effective pharmaceutical composition or a dosage regimen for preventing and / or treating bleeding, a disease accompanying bleeding, or a disease caused by bleeding. The inventors discovered that by administering a pharmaceutical composition comprising a bispecific antigen-binding molecule that recognizes (a) blood coagulation factor IX and / or activated blood coagulation factor IX and (b) blood coagulation factor X and / or activated blood coagulation factor X according to a given dosage regimen, bleeding, a disease accompanying bleeding, or a disease caused by bleeding can be prevented and / or treated more effectively.
Owner:F HOFFMANN LA ROCHE & CO AG +1
Methods of using a bispecific antibody that recognizes coagulation factor IX and/or activated coagulation factor IX and coagulation factor X and/or activated coagulation factor X
ActiveUS11352438B2Reduce morbidityImmunoglobulins against blood coagulation factorsAntibody ingredientsDosing regimenAntigen
An objective of the present invention is to provide an effective pharmaceutical composition or a dosage regimen for preventing and / or treating bleeding, a disease accompanying bleeding, or a disease caused by bleeding. The inventors discovered that by administering a pharmaceutical composition comprising a bispecific antigen-binding molecule that recognizes (a) blood coagulation factor IX and / or activated blood coagulation factor IX and (b) blood coagulation factor X and / or activated blood coagulation factor X according to a given dosage regimen, bleeding, a disease accompanying bleeding, or a disease caused by bleeding can be prevented and / or treated more effectively.
Owner:F HOFFMANN LA ROCHE & CO AG +1
Coagulation factor ⅸ quality control product preparation method
InactiveCN104181313BEasy to detectImprove uniformityPreparing sample for investigationBiological testingFreeze-dryingBlood plasma
The invention relates to a preparation method of a clinical blood coagulation inspection preparation and particularly relates to a preparation method of a blood coagulation factor IX quality control product. The preparation method comprises the following steps: carrying out affinity chromatography on the mixed blood plasma of multiple persons by using an anti-human blood coagulation factor IX monoclonal antibody immunoaffinity chromatography column, removing a blood coagulation factor IX in the mixed blood plasma of multiple persons to obtain a blood plasma in shortage of the blood coagulation factor IX; mixing the mixed blood plasma of multiple persons with the blood plasma in shortage of the blood coagulation factor IX according to a certain proportion to prepare a blood coagulation factor IX quality control product with the content of the blood coagulation factor IX at different concentration levels, adding a freeze-drying protective additive, carrying out sub-packaging, and carrying out freeze drying so as to obtain the blood coagulation factor IX quality control product. According to the blood coagulation factor IX quality control product prepared by the preparation method, the uniformity, the stability and the stability of freeze-dried aquatic product subjected to re-melting are good, and the quality control product can replace an imported product to be used for quality control on detection of blood coagulation factor IX, so that the reduction of the detection cost is facilitated and the capability of detecting the blood coagulation factor IX in China can be promoted.
Owner:BLOOD TRASFUSION INST CHINESE ACAD OF MEDICAL SCI +1
A method for simultaneously separating and purifying blood coagulation factors ix, x and ⅶ from human plasma
ActiveCN109651502BAchieve separationEasy to operateFactor VIIPeptide preparation methodsUltrafiltrationAnion-exchange chromatography
The invention discloses a method for simultaneously separating and purifying coagulation factors IX, X and VII from human plasma, which comprises the following steps: performing centrifugal impurity removal, gel adsorption and ultrafiltration concentration to prepare a prothrombin compound; separating the coagulation factors VII and a mixed solution containing IX and II through an anion exchange resin column; separating the coagulation factor II and IX from the mixed solution containing IX and II by affinity chromatography. According to the method, by combining anion exchange chromatography with heparin affinity chromatography, the separation and preparation of three coagulation factors II, VII and IX at the same time can be achieved; the method has the advantages of high raw material utilization, simple operation and short time consumption; meanwhile, by detecting the electric signals in the chromatography process, the corresponding coagulation factors are accurately collected, the purity of the coagulation factors is effectively improved, and the economic benefit is improved.
Owner:HUALAN BIOLOGICAL ENG INC +2
Stable compositions of factor ix
InactiveUS20130149293A1Improve stabilityAvoid damagePeptide/protein ingredientsPharmaceutical delivery mechanismFactor iiNuclear chemistry
The invention allows substantial improvements in stability of coagulation Factor IX in aqueous compositions. An aqueous composition sealed in a non-glass container comprising Factor IX in a buffer and calcium ions is provided, together with methods of stabilizing an aqueous Factor IX composition comprising storing said composition in a non-glass container for at least 7 days.
Owner:ARECOR LTD
Method for purifying vitamin k-dependent proteins such as coagulation factor ix
A method for the preparation of prion-free vitamin K-dependent proteins in a purification sequence using chromatography, characterized in that: - at least one chromatographic step is performed using a multimodal resin; - vitamin K-containing dependent proteins are provided as an aqueous solution fraction of protein; - contacting fraction containing vitamin K-dependent protein with multimodal resin at pH 6-9; - optional washing with aqueous wash buffer prior to elution of vitamin K-dependent protein Multimodal resin for vitamin K-dependent proteins to wash away contaminants and retain vitamin K-dependent proteins; -Elute vitamin K-dependent from multimodal resins at pH 6-9 in arginine-containing buffers protein; and - optionally collecting the vitamin K-dependent protein-containing fraction in purified or enriched form.
Owner:OCTAPHARMA
Coagulation Factor IX Conjugates
InactiveUS20170035890A1Extended half-lifeProlong half-life in vivoPeptide/protein ingredientsEnzyme stabilisationPolymerHaemophilia B
Owner:NOVO NORDISK HEALTH CARE AG
Method for manufacturing high purified factor IX
ActiveCN101291951BPeptide preparation methodsPeptidasesCell culture mediaAnion-exchange chromatography
The present invention relates to a method for manufacturing a highly purified human blood coagulation factor IX, including: subjecting objects containing human blood coagulation factor IX (derived from human plasma or recombinant cell culture medium) to anion exchange chromatography, cation exchange chromatography, heparin affinity chromatography, preparing the human blood coagulation factor IX, and including: subjecting the S / D treatment (Solvent / Detergent treatment) to inactive viruses or removing the viruses through nanofiltration, thereby obtaining a highly purified, safe coagulation factor IX having a specific activity of above 150 IU / mg, with substantially inactivation or removal of all impure proteins and viruses.
Owner:THE GREEN CROSS CORP
Urine blood coagulation factor IX and application of polypeptide fragment thereof in burns
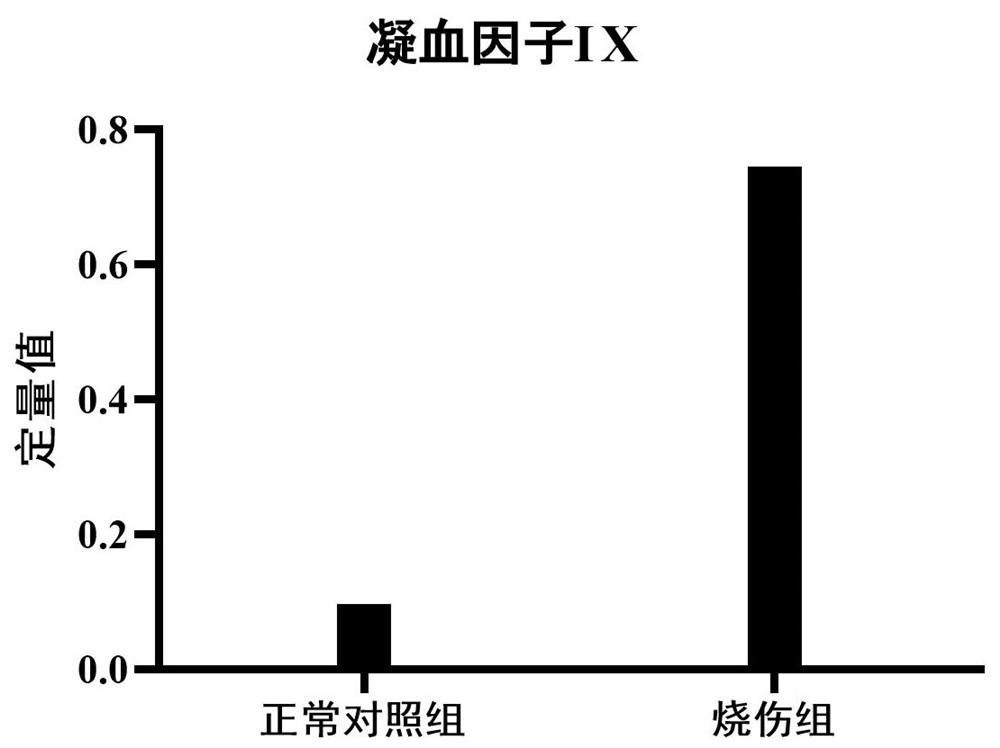
PendingCN114113640AEasy to storeMicrobiological testing/measurementMaterial analysis by electric/magnetic meansURINE BLOODSevere burn
The invention provides application of a urine coagulation factor IX and a polypeptide fragment of the urine coagulation factor IX, and particularly relates to application of the urine coagulation factor IX and the polypeptide fragment of the urine coagulation factor IX in preparation of preparations for burn diagnosis, differential diagnosis, burn area and degree evaluation, treatment effect evaluation, monitoring, prognosis evaluation, mechanism research and the like. Burns are common important wounds in daily life, about 5000-100000 people in every one million people are burnt every year, according to statistics of World Health Organization, more than 300,000 people die from burn patients globally every year, and the treatment survival rate of serious burns is still at a lower level. Researches prove that compared with healthy people (a normal control group), the expression of the urine blood coagulation factor IX and the polypeptide fragment thereof in burn patients is increased, and the content of the urine blood coagulation factor IX and the polypeptide fragment thereof is gradually increased along with the aggravation of the burn degree. The method can be used for various purposes of application detection of burn patients. The advantages of noninvasive acquisition, large-scale repeated sampling and convenient preservation of a urine sample are exerted, and the urine blood coagulation factor IX and the polypeptide fragment thereof are detected by utilizing the urine sample.
Owner:张曼
A method for adsorbing human prothrombin complex from plasma
ActiveCN104109202BAchieve short timeHigh yieldPeptide preparation methodsPeptidasesProthrombin complex concentrateCellulose
The invention relates to a production method for adsorbing a human complex from plasma by a fixed bed column chromatography technique, which comprises the following steps: (1) cryoprecipitation plasma removal: filtering by using a cellulose deep filter plate which is cleaned by an EDTA (ethylene diamine tetraacetic acid) solution and a sodium citrate solution; (2) filtering the plasma subjected to deep filtration through a 0.2 mu m filter element membrane while fixed bed loading; (3) balancing 2-5 column volumes in a fixed bed chromatographic column filled with anion exchange gel Capto DEAE by using a buffer solution A at the plasma loading flow rate of 60-120 cm / hour, washing the chromatographic column with a buffer solution B, and eluting the chromatographic column with a buffer solution C to obtain a PCC (prothrombin complex concentrate) product. When the calculation is based on coagulation factor IX, the yield of the PCC can reach 75-90%, and the specific activity can reach 5.5 IU / mg above.
Owner:SHANDONG TAIBANG BIOLOGICAL PROD CO LTD
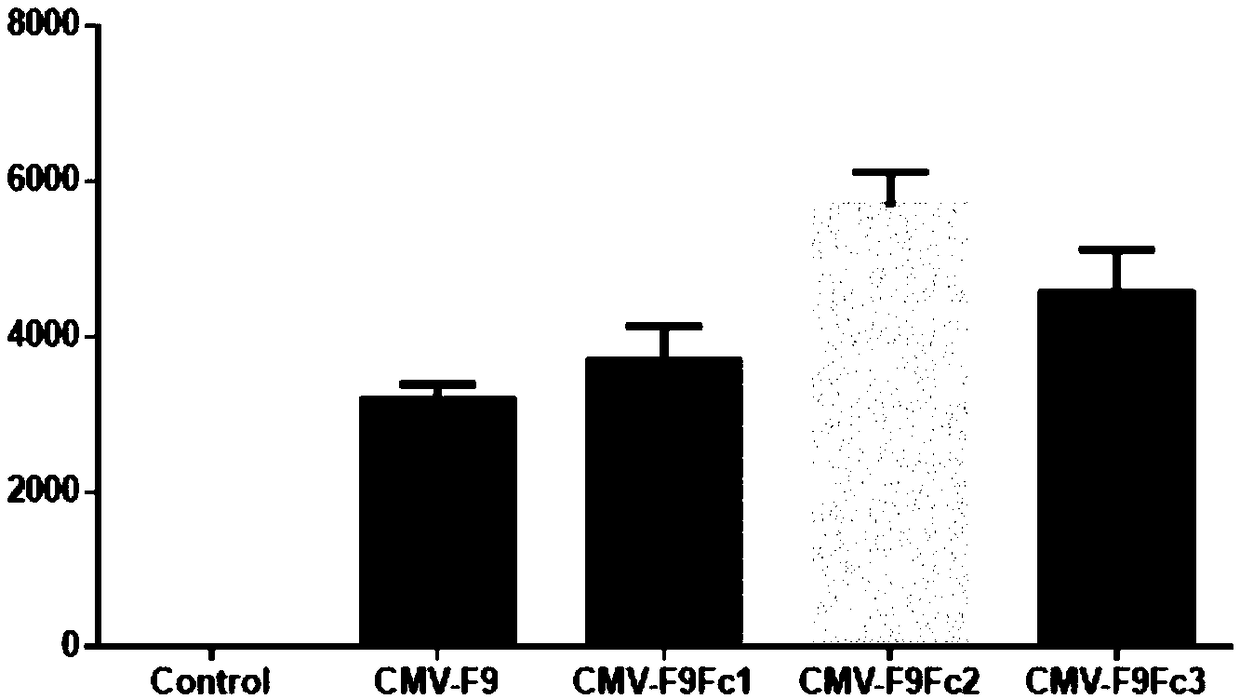
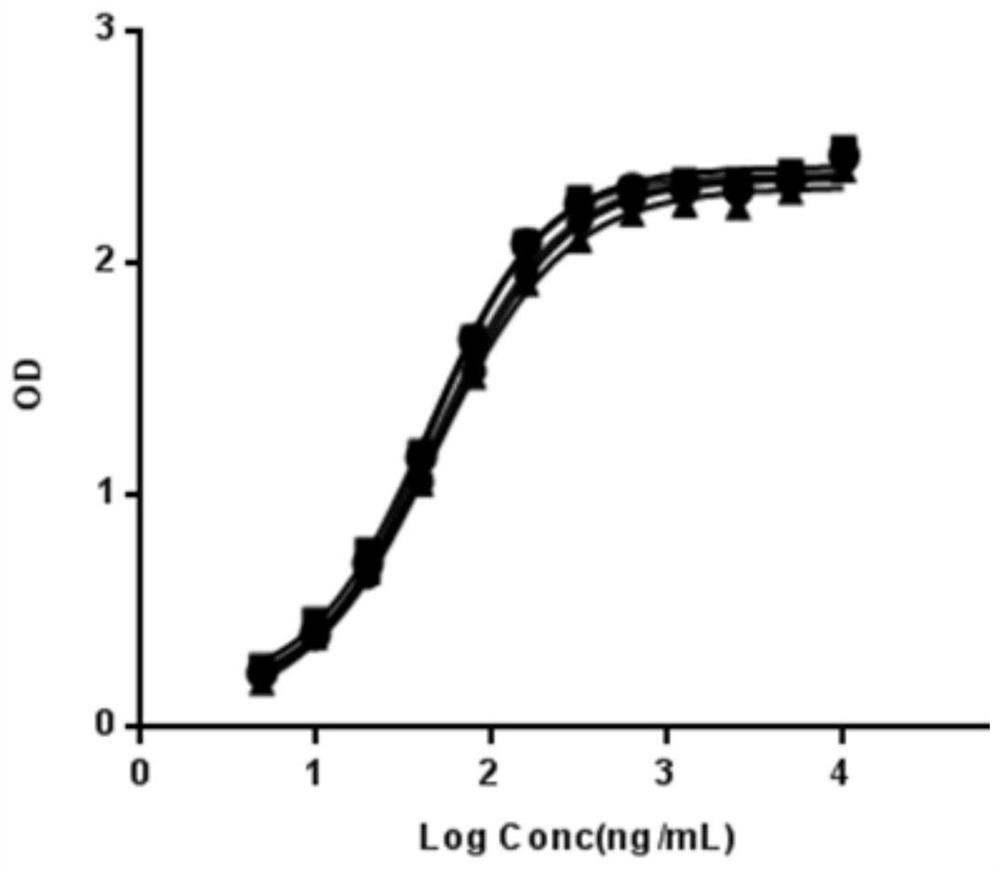
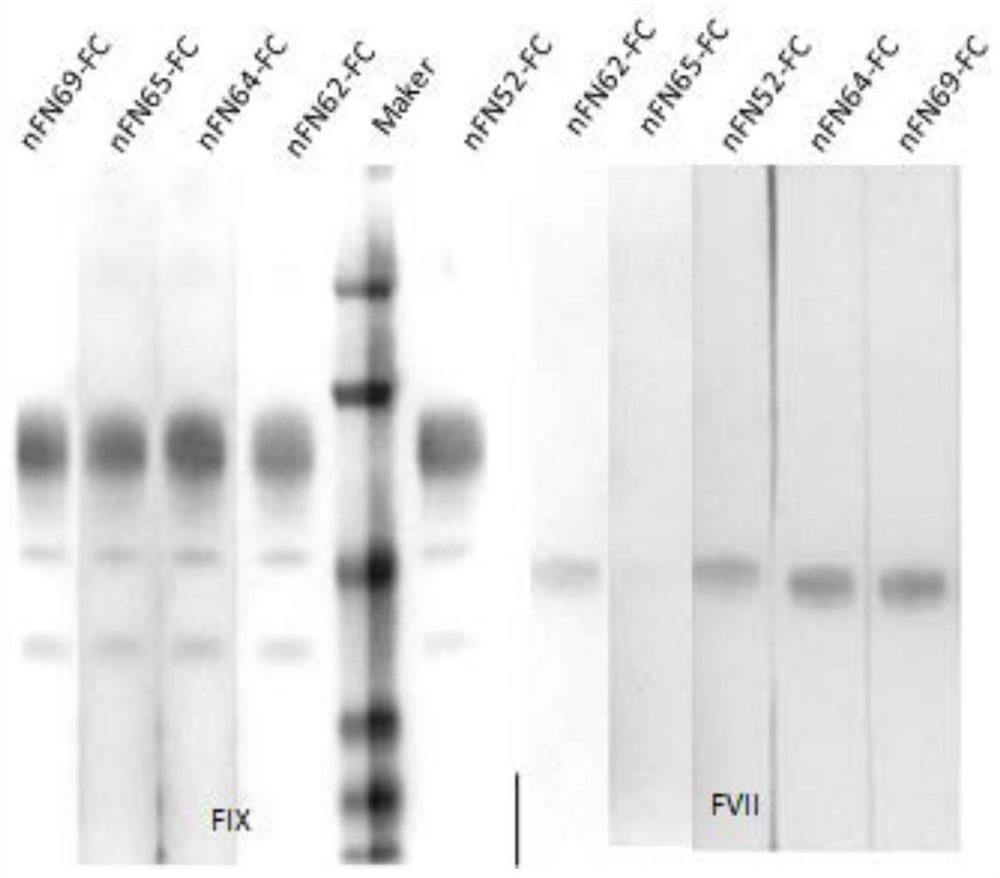
High-activity recombinant human coagulation factor IX fusion protein and preparation method thereof
InactiveCN109439679AHigh specific activityAntibody mimetics/scaffoldsHybrid peptidesHuman immunoglobulinsMammalian genome
The invention discloses a high-activity recombinant human coagulation factor IX fusion protein and a preparation method thereof. The high-activity recombinant human coagulation factor IX fusion protein comprises a promoter, a human coagulation factor IX protein gene, a human immunoglobulin IgG1 Fc fragment gene and a terminator. A carrier is used to transfer a human FIX-Fc fusion protein gene expression kit to mammalian genome to obtain a transgenic animal; human FIX-Fc fusion expression protein is attained from milk of the transgenic animal. According to the method herein, the human FIX expressed in the milk of the animal may reach 6 IU / ml in activity and 3000 IU / mg in average specific activity which is about 12 times as high as that of natural FIX, and is applicable to the production ofhuman coagulation factor IX.
Owner:SHANGHAI CHILDRENS HOSPITAL +1
Single domain antibody against coagulation factor ix(fix)
ActiveCN110343181BImmunoglobulins against blood coagulation factorsPreparing sample for investigationSingle-domain antibodyBlood plasma
The invention relates to the field of medicine and biology, and discloses a single-domain antibody against blood coagulation factor IX (FIX). Specifically, the present invention discloses a FIX-binding molecule derived from the single-domain antibody and its use, especially for detecting FIX and preparing FIX-deficient plasma.
Owner:SUZHOU ALPHAMAB
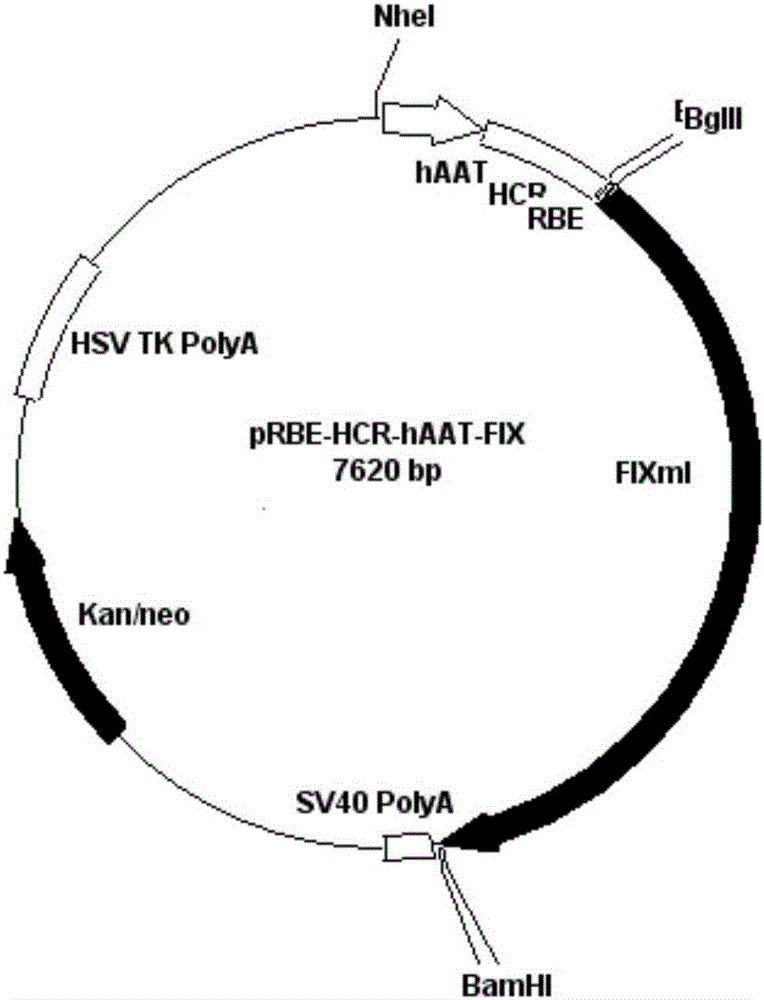
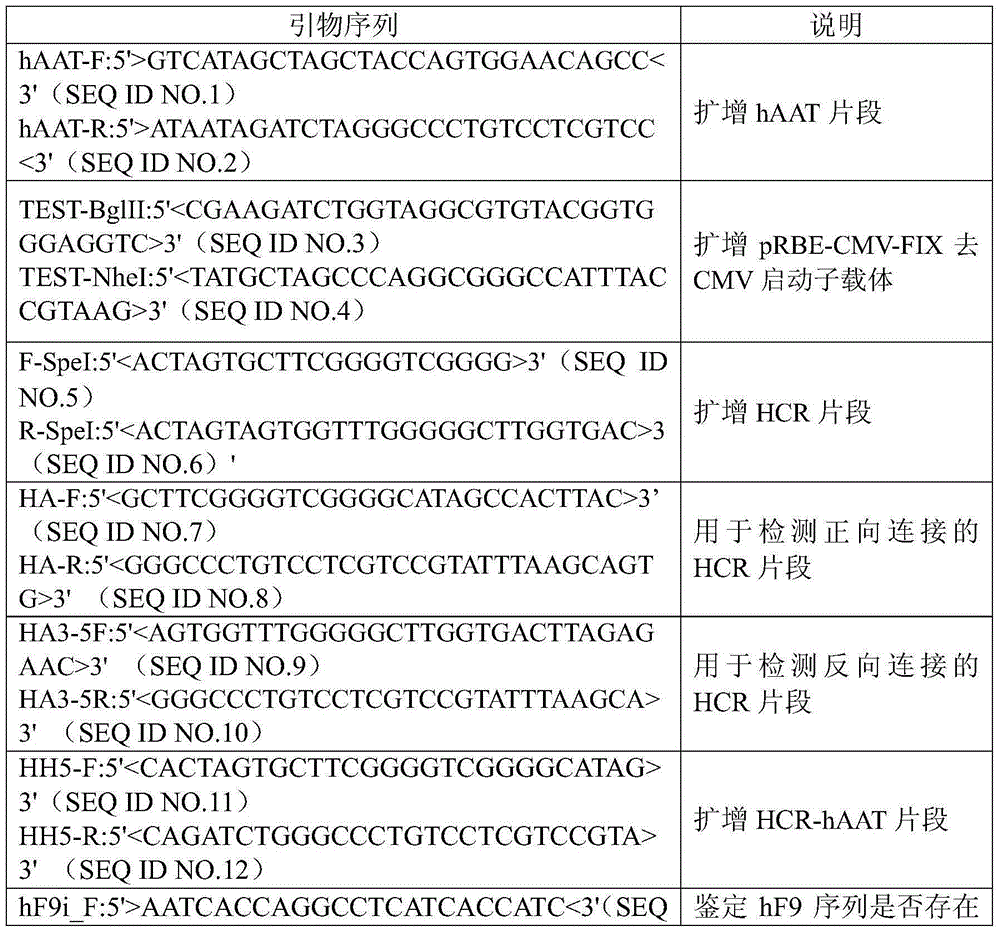
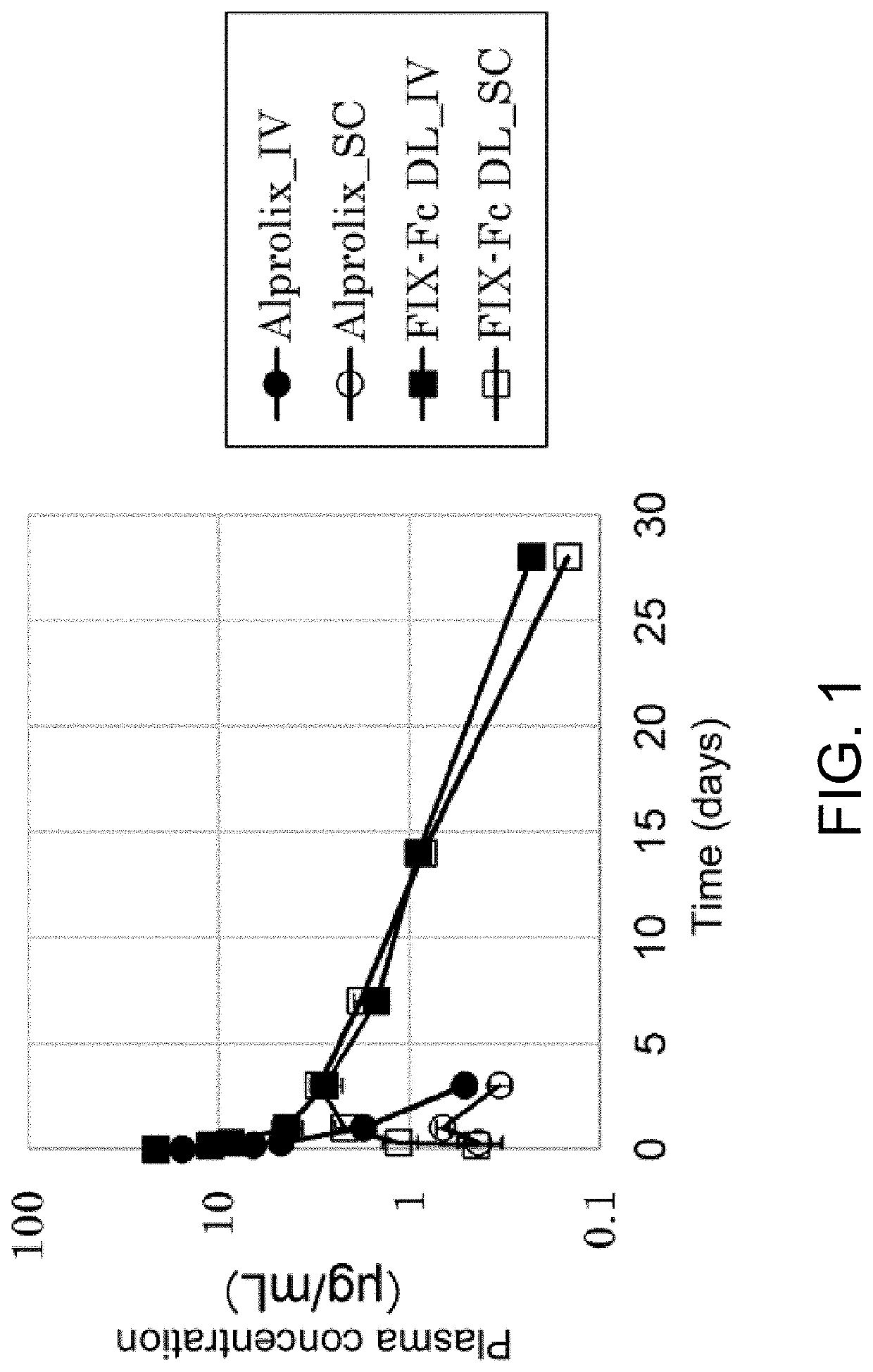
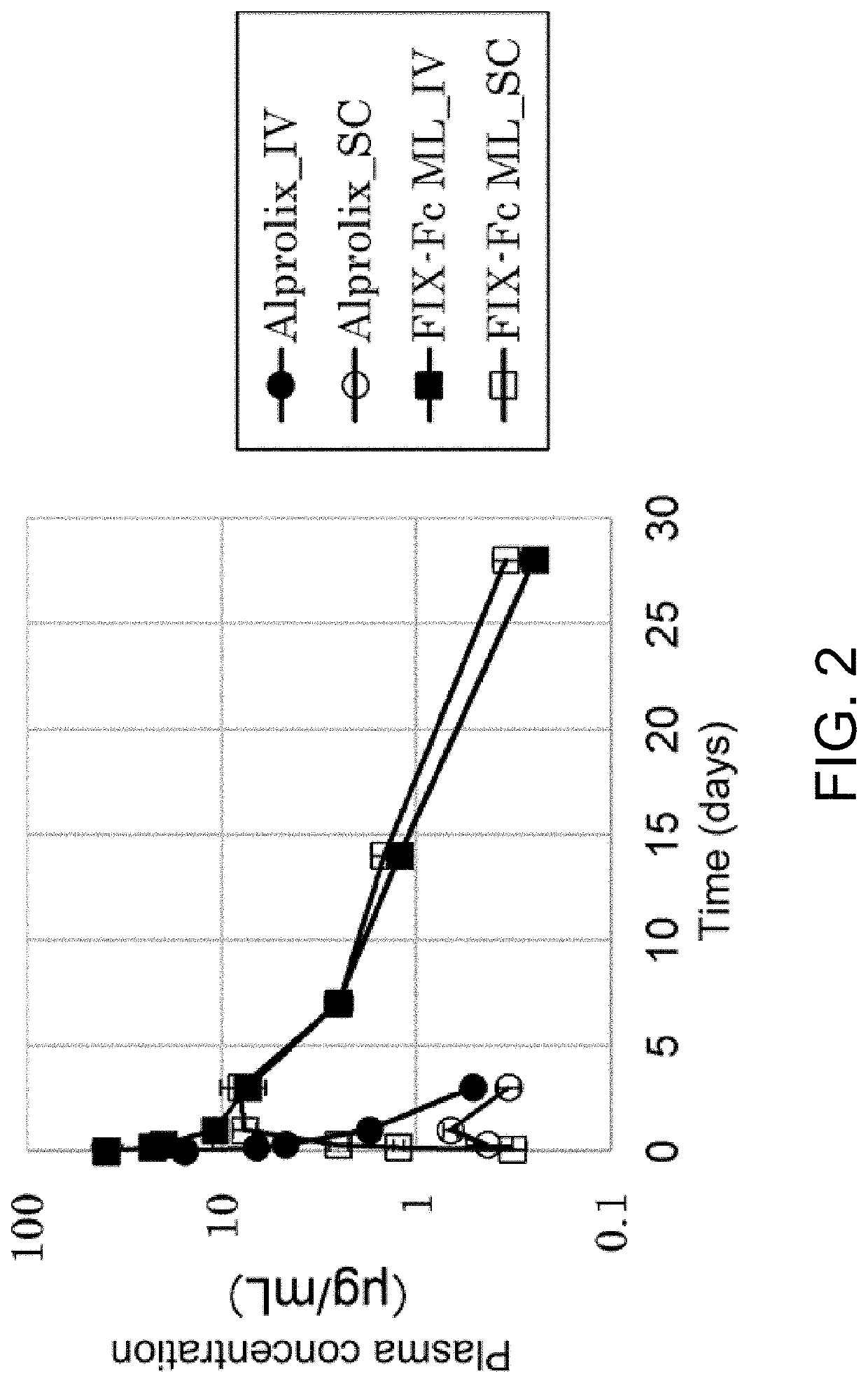
A kind of prbe-hcr-haat-hfixml plasmid and its construction and application
InactiveCN103667346BHigh expressionExtend the time of expressionVector-based foreign material introductionHigh pressureChromosome 19
The invention relates to pRBE-HCR-hAAT-hFIXml plasmid as well as construction and application thereof. The sequence of the plasmid is represented as SEQ ID NO.17, and the plasmid contains an hAAT promoter, an RBE (rep binding element) and an HCR (hepatic locus control region) control element. The plasmid and rep expression plasmid are injected into a mammal body at high pressure and can be used for targeted integration of an exogenous gene onto a specific site of AAVS1 (adeno-associated virus integration site1) on the No.19 chromosome of a human so as to express a coagulation factor IX of the human. With application of the expression plasmid, the coagulation factor IX of the human can be expressed stably, and the integration efficiency of an integration system in a liver is improved, so that higher expression quantity and longer expression time are achieved.
Owner:YANGZHOU UNIV
Coagulation factor ix with improved pharmacokinetics
PendingUS20200109390A1Prolonged plasma half-lifeExtended half-lifeImmunoglobulins against blood coagulation factorsPeptide/protein ingredientsAntibody fragmentsBULK ACTIVE INGREDIENT
The present invention provides a method for improving or controlling the plasma half-life and / or bio-availability of blood coagulation factor IX (FIX), the method comprising modifying the GLA domain. Examples of such modifications include: (i) non-covalent bonding of a GLA-domain-recognizing antibody or an antibody fragment thereof to the GLA domain; (ii) reduced number of Gla residues in the GLA domain, in comparison to that of a native FIX; (iii) either or both of deletion of one or more glutamic acid residues in the GLA domain and substitution of one or more glutamic acid residues in the GLA domain with another amino acid; and (iv) deletion of a part or all of the GLA domain. The present invention also provides a FIX with improved pharmacokinetics which carries such modifications, a pharmaceutical composition containing the FIX as an active ingredient, a method for producing the FIX, and such.
Owner:CHUGAI PHARMA CO LTD
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com