High-activity recombinant human coagulation factor IX fusion protein and preparation method thereof
A human blood coagulation factor and fusion protein technology, which is applied in the field of highly active recombinant human blood coagulation factor IX fusion protein and its preparation, can solve the problems of unknown high expression level and achieve high specific activity and high-efficiency expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
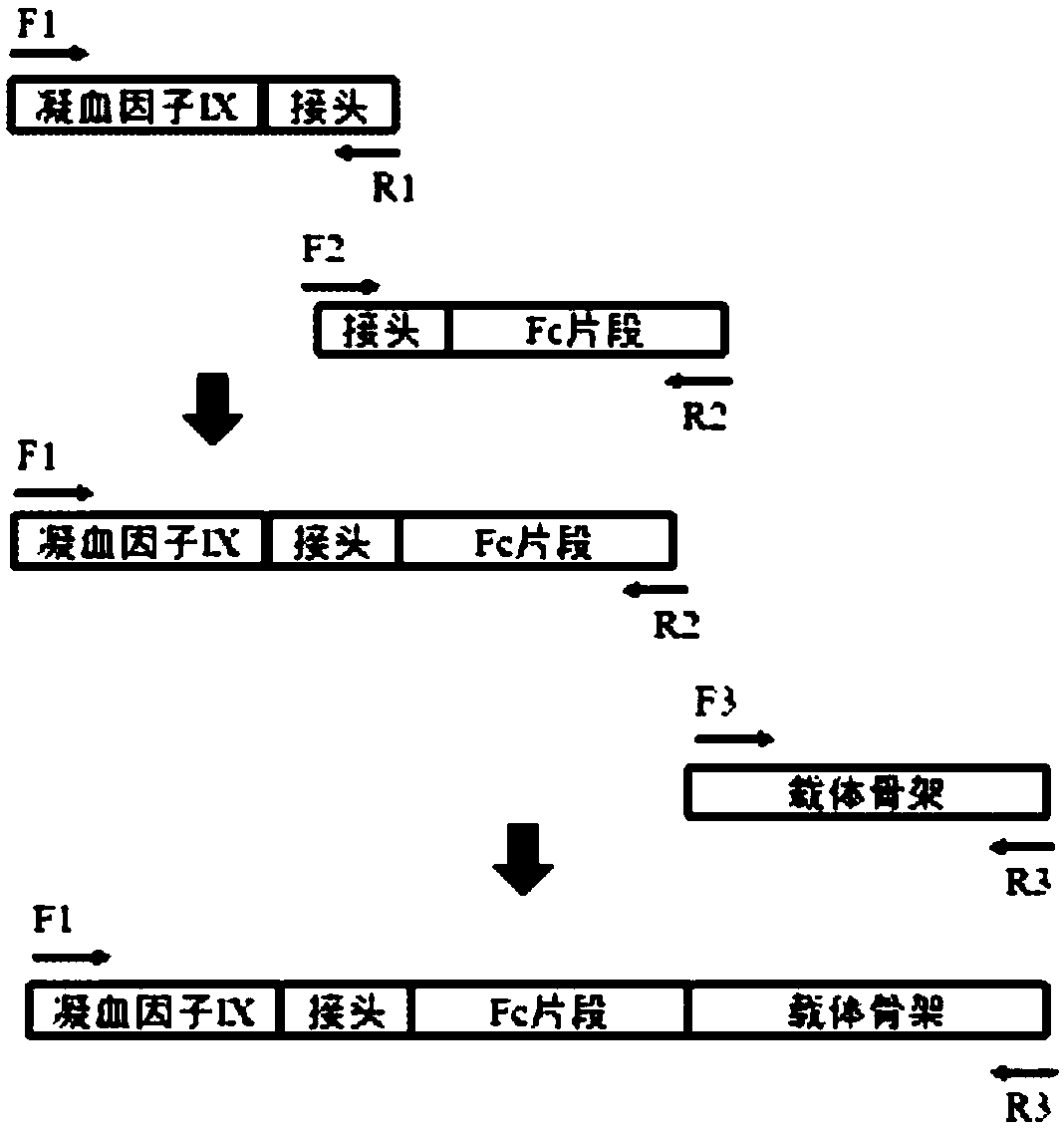
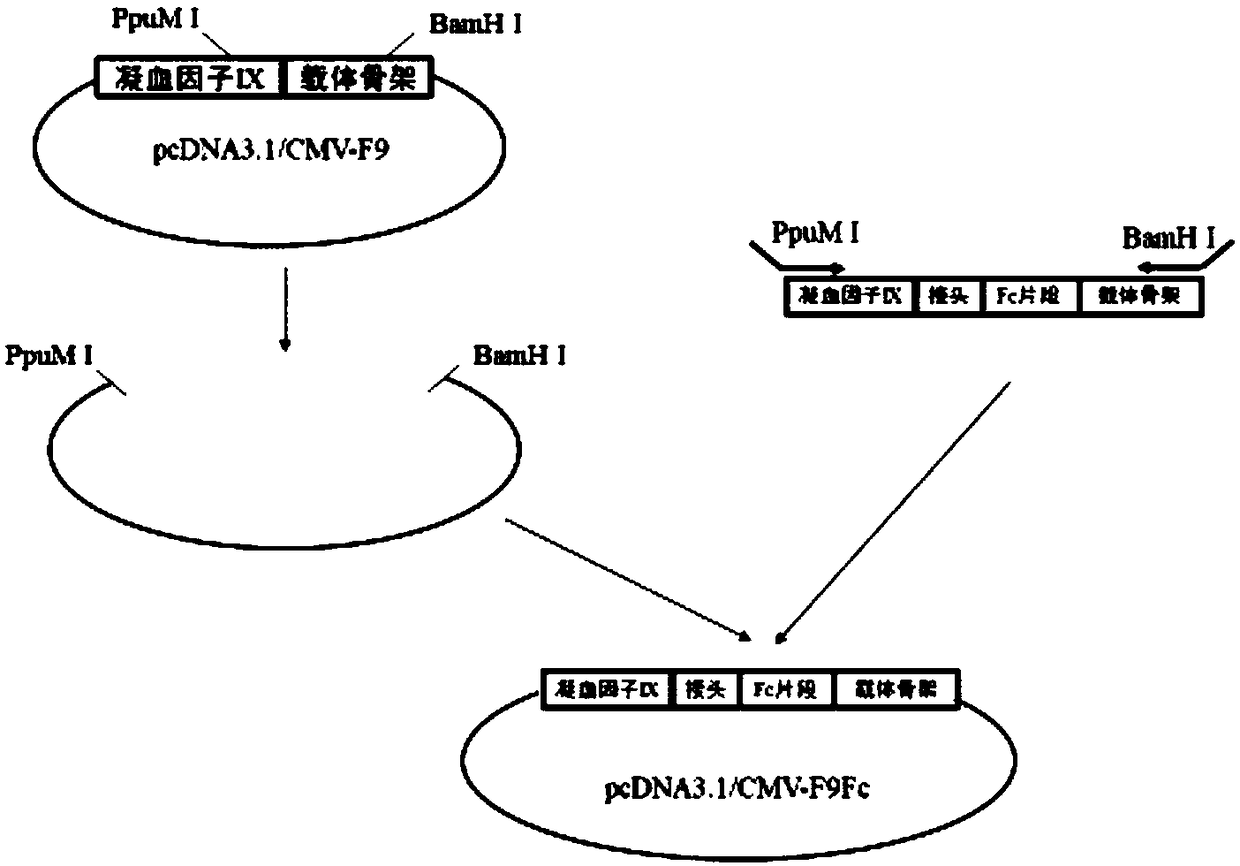
Embodiment 1
[0030] 1. Fragment synthesis
[0031] 1) Amplification of human IgG1Fc cDNA fragment
[0032] The upstream primer Fc-F: 5'-GAGCCCAAATCTTGTGACAAAACT-3'; the downstream primer Fc-R: 5'-TTTACCCGGAGACAGGGAGAGGCT-3' were used to perform PCR amplification using human peripheral blood cell cDNA as a template. The reaction system is as follows:
[0033]
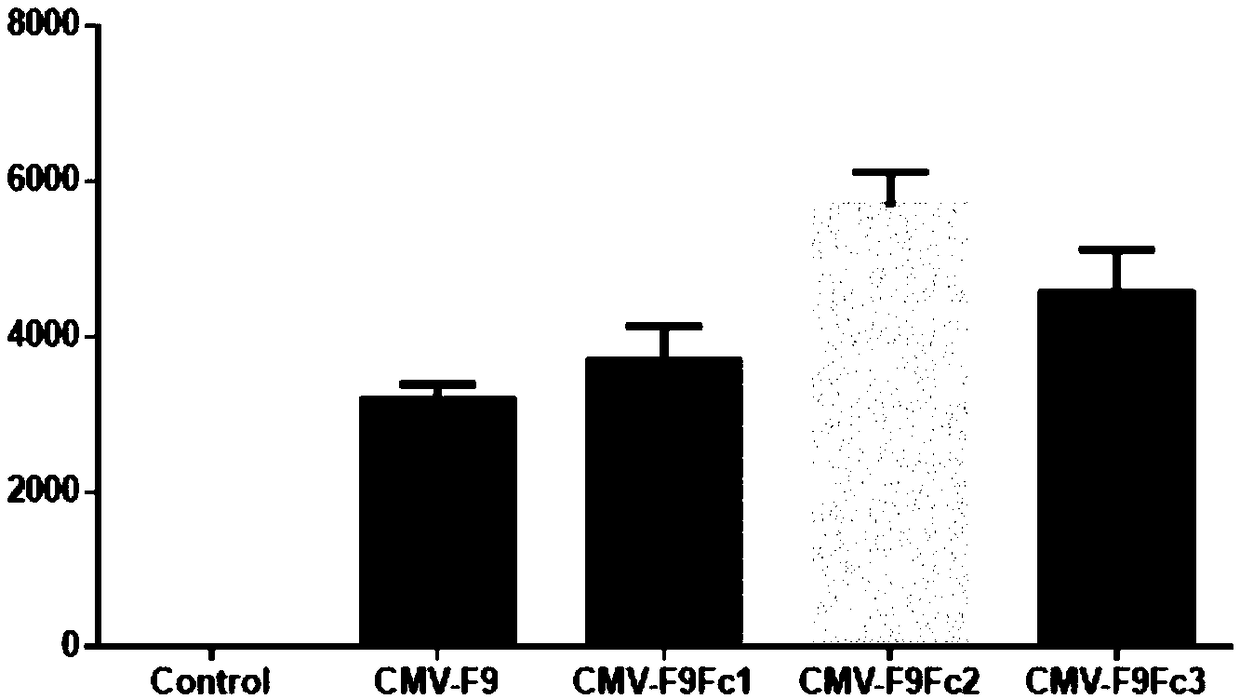
[0034] A total of 4 reaction tubes are made, and the reaction conditions are 94℃5min; (94℃45sec, 56℃45sec, 72℃3min)×30 cycles; 72℃10min. Amplify about 0.7kb hIgG1Fc cDNA fragment, mix the reaction solution, take 2μL and send it to Shanghai Meiji Biotechnology Company for sequencing, the remaining products are subjected to agarose electrophoresis, gel cut, and the 0.7kb hIgG1Fc cDNA fragment is recovered with the kit. Then the sequence obtained by sequencing was compared with the sequence AF150959.1 in the Genebank database, and the comparison found that there was a difference of 1 base. It was verified that the site was a SNP site and did...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific activity | aaaaa | aaaaa |
| Specific activity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com