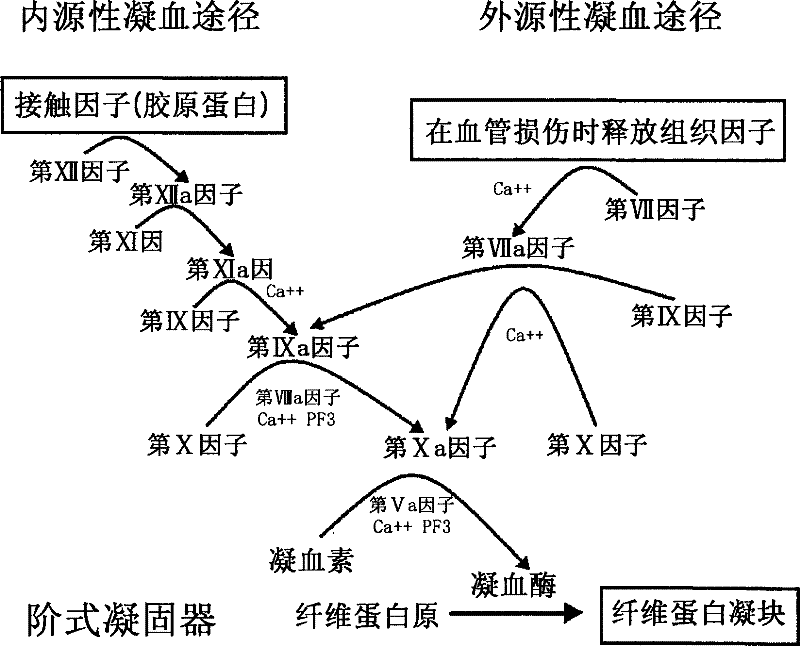
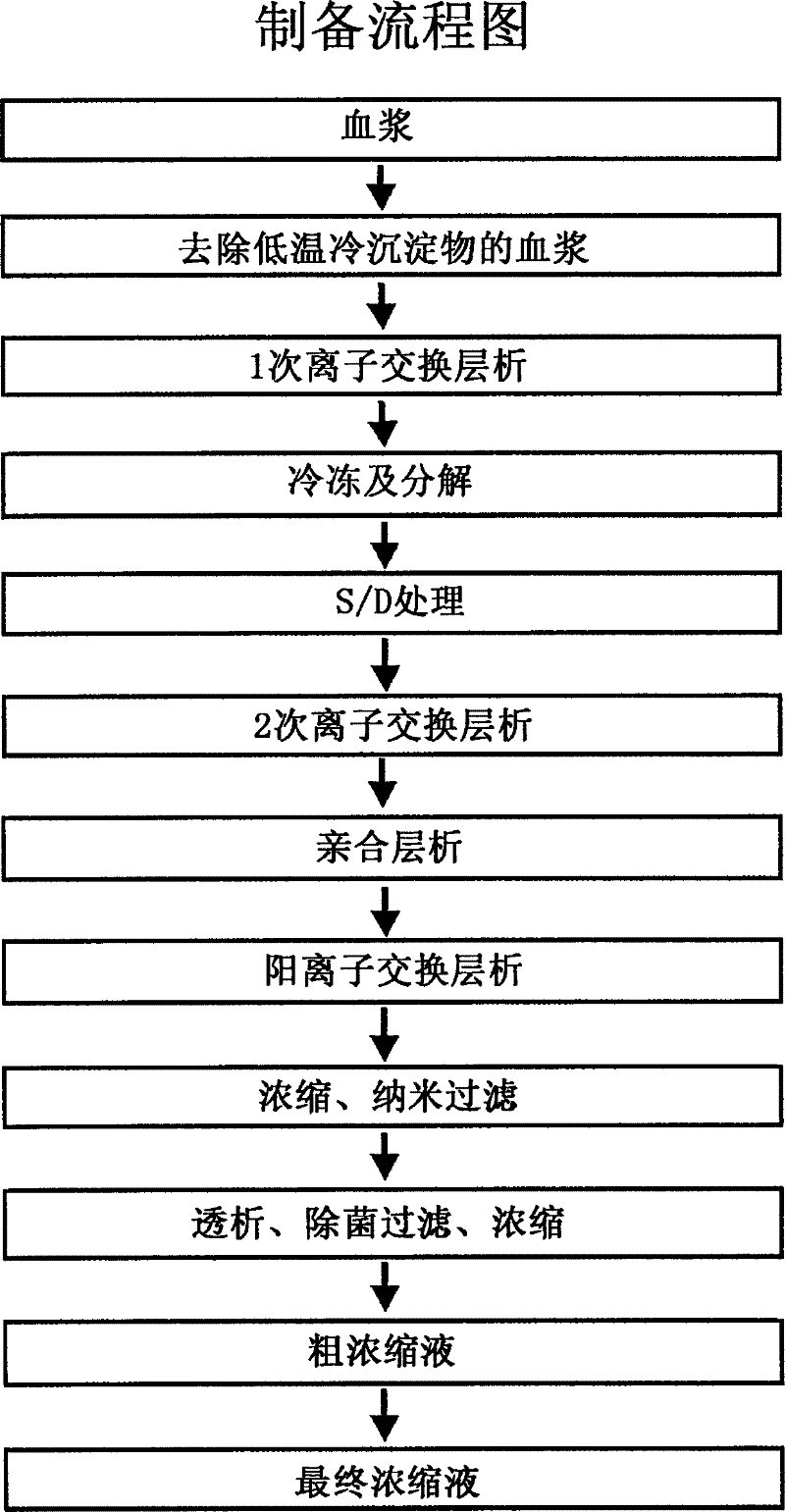
Method for manufacturing high purified factor IX
A blood coagulation factor and factor technology, which is applied in the field of preparation of human coagulation factor IX, can solve the problems that the virus cannot be completely overcome, the virus infection cannot be ruled out, and the side effects cannot be ruled out.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Purification by anion exchange chromatography (traditional procedure)
[0027] Using the plasma of the decondensed protein or the cell culture medium of recombinant factor IX as the raw material, the raw material is mixed with diethylamino-ethyl-glucose gel A-50 (DEAE-Sephadex A-50), and pre-washed with buffer (prewash), it stirred at 4 degreeC temperature conditions for 2 hours. Then, the gel adsorbed with factor IX is filtered and separated by centroids. After washing, Factor IX is eluted by buffers with high salt concentration. The pH of the washing solution was appropriately adjusted to about 7.5, followed by dialysis, concentration, and storage at -70°C. In addition, the concentrate was separately sampled (aliquot), and the concentration was measured according to the standards and experimental methods for blood preparations and reconstituted preparations prescribed by KFDA (Korea Food and Drug Administration). The virus purification process is complet...
Embodiment 2
[0028] Example 2: Purification using Heparin sepharose 6FF column chromatography
[0029] After adjusting 10 ml of the IX coagulation factor complex solution purified and prepared by the above Example 1 to an ionic strength of 20 mS / cm or less and a pH of 7.0, it was placed on a heparin sepharose 6FF column (column conditions: diameter 1 cm, height 18 cm, Flow rate (flow rate: 1.0 ml / min, normal temperature) was flowed into an equilibration buffer (20 mM sodium citrate, pH 7.0) to maintain equilibrium, and a loading sample was flown through to adsorb, by the same amount as the equilibration buffer. The solution was washed once with 0.1M NaCl, followed by a second wash with 0.25M NaCl. The protein concentration of the eluent was measured, followed by SDS-PAGE (BSA standard (2 mg / ml, PIERCE)), and the concentration was measured using Coagulation timer KC10 from Amelung. Comparing the specific activity of the second anion column washing solution of Example 1 and the eluent of th...
Embodiment 3
[0030] Example 3: Purification by Cation Exchange Chromatography (CM-sepharose FF)
[0031] After adjusting 10ml of the IX coagulation factor complex purified by the above Example 2 to an ionic strength of 20mS / cm or less and pH 4.0, it is suitable for equilibration buffer (0.30-0.4M sodium chloride in citrate accounts for 20mM). , pH 4.0) to maintain an equilibrium CM-sepharose FF column (column conditions: diameter 1 cm, height 5 cm, flow rate 1.0 ml / min), and the non-adsorbed liquid was collected. The protein concentration of the above-mentioned non-adsorbed liquid was measured and then SDS-PAGE was carried out, and the lowest specific activity was found to be 150 IU / mg or more.
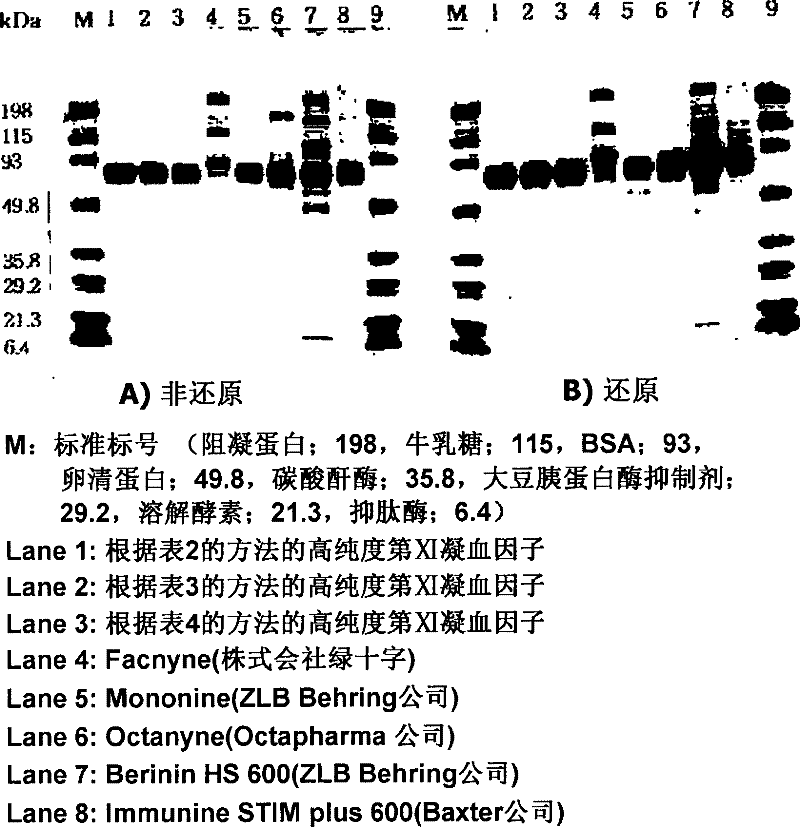
[0032] image 3 It is a schematic diagram showing the results of SDS-PAGE for confirming the purity of the XI coagulation factor preparation prepared by the present invention and other products on the market.
[0033] like image 3 As shown, the IX coagulation factor preparation purified and pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com