A method for adsorbing human prothrombin complex from plasma
A technology of human prothrombin and complexes, which is applied in the production of biological products and blood products, can solve the problems of gel leakage operation, cross-contamination, pollution, etc., achieve high flow rate, avoid pollution, and improve the yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
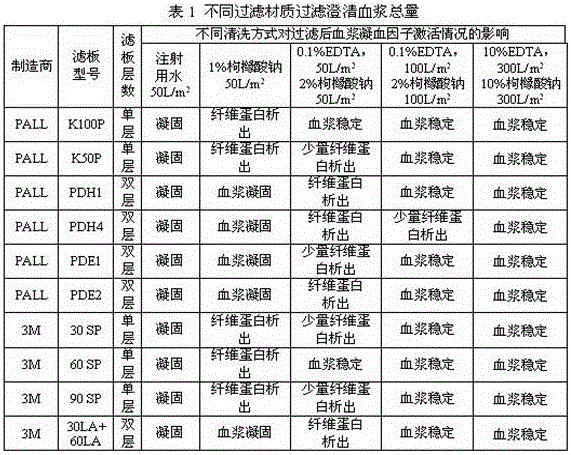
[0026] Example 1: Deep plasma filtration
[0027] Choose different filter plates and corresponding fixtures for assembly. Clean according to the cleaning method in the table below, and place the filtered plasma at 2~8°C for 24 hours to observe the coagulation of the plasma.
[0028]
Embodiment 2
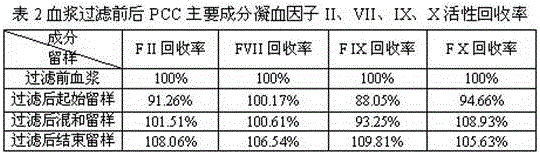
[0029] Example 2: Verification of the recovery rate of human coagulation factor activity in plasma after filtration
[0030] Use 3M’s 90SP (14cm 2 )According to 10%EDTA 300L / m 2 , 10% sodium citrate 300L / m 2 After the method is cleaned, 200ml of plasma is filtered, and the coagulation factors II, VII, IX, X in the liquid before and after filtration are detected.
[0031]
[0032] The above results show that the use of filter plates treated with a filter plate balance solution containing sodium citrate components after plasma filtration did not cause the activity of the main components of the human prothrombin complex to coagulation factors II, VII, IX, and X. Activation or deactivation. Therefore, the filtered and clarified plasma can be used for the separation and purification of human prothrombin complex by fixed bed column chromatography.
Embodiment 3
[0033] Example 3: Separation and purification of PCC by fixed bed column chromatography
[0034] Load 1L Capto DEAE gel into a fixed-bed chromatography column, and use buffer A (25mmol / L sodium citrate, 20mmol / L arginine hydrochloride buffer, pH7.0) to equilibrate 2~5 column volumes. After deep filtration, 30L of plasma is filtered through a 0.2μm in-line membrane and pumped into the chromatography column. The sample flow rate is 60~120cm / h. Then wash the column with buffer A containing 160 mmol / L NaCl, and collect the flow-through and washing solution for the separation and purification of other proteins. Finally, the column was eluted with buffer A containing 500 mmol / L of NaCl to obtain PCC products.
[0035]
[0036] Among them, the specific activity of factor IX can reach more than 5.5IU / mg, which is more than 10 times that of the PCC produced by the traditional DEAESephadex A50 gel batch adsorption process.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com