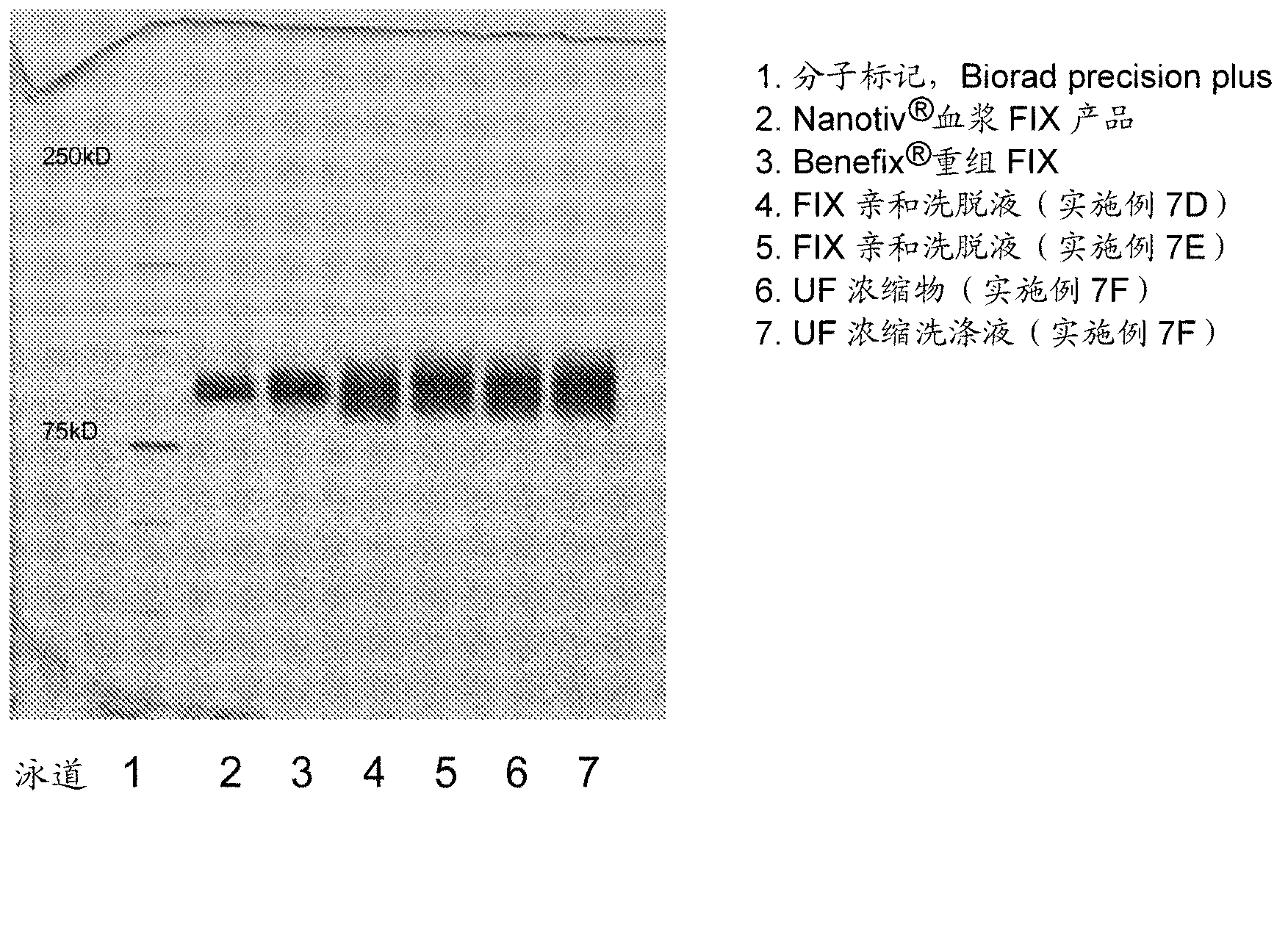
A process for purifying vitamin k dependent proteins such as coagulation factor IX
A vitamin-dependent technology, applied in coagulation/fibrinolytic factors, peptide preparation methods, chemical instruments and methods, etc., can solve expensive problems, achieve the effect of inhibiting aggregates and maintaining natural molecular structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
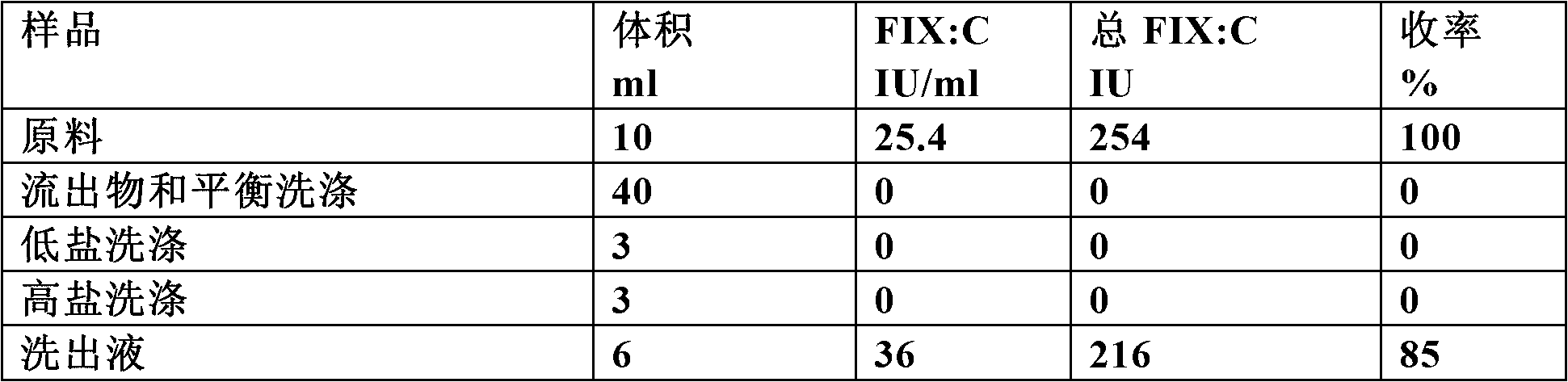
[0116] raw material
[0117] Using Bloodborne FIX (pdFIX, ). Lyophilized Nanotiv was dissolved and diluted in equilibration buffer prior to loading onto Capto MMC columns.
[0118] Chromatography Resins and Columns
[0119] The mixed-mode resin Capto MMC (cat number 17-5317) from GE Healthcare was used as the capture step for the FIX molecules. Capto MMC is a weak cationic resin with hydrophobic and thiophilic interactions and hydrogen bonding. A Tricorn 5 / 150 column was packed with Capto MMC resin to a bed height of 15.7 cm. The column volume (CV) was 3.1 ml.
[0120] buffer
[0121] Equilibration buffer: 20mM sodium citrate, 0.1M NaCl, 0.02% Polysorbate 80, pH 7.0
[0122] Low Salt Wash Buffer: 20mM Sodium Citrate, 0.2M NaCl, 0.02% Polysorbate 80, pH 6.5
[0123] High Salt Wash Buffer: 20mM Sodium Citrate, 0.7M NaCl, 0.02% Polysorbate 80, pH 6.5
[0124] Elution buffer: 20mM sodium citrate, 0.2M NaCl, 0.5M arginine hydrochloride, 0.02% Polysorbate80, pH 6.5
[012...
Embodiment 2
[0133] raw material
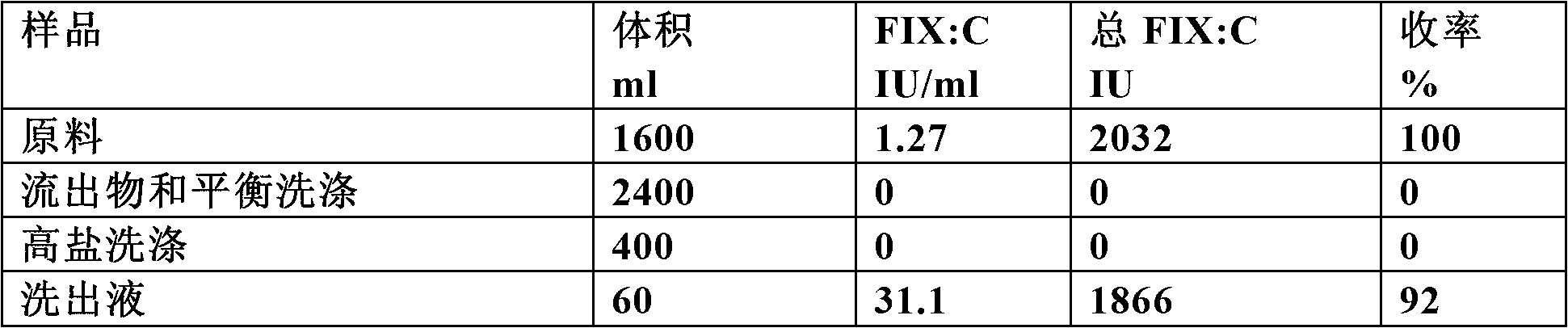
[0134] Recombinant human FIX (rhFIX) was produced in HEK 293 cells. Cells are removed and the cell-free supernatant is the starting material for loading onto a Capto MMC column.
[0135] Chromatography Resins and Columns
[0136] The mixed-mode resin Capto MMC (cat number 17-5317) from GE Healthcare was used as the capture step for the FIX molecules. Capto MMC is a weak cationic resin with hydrophobic and thiophilic interactions and hydrogen bonding. The XK26 column was packed with Capto MMC resin to a bed height of 15 cm. The column volume (CV) was 80ml.
[0137] buffer
[0138] Equilibration buffer: 20mM sodium citrate, 0.1M NaCl, 0.02% Polysorbate 80, pH 7.0
[0139] High Salt Wash Buffer: 20mM Sodium Citrate, 0.7M NaCl, 0.02% Polysorbate 80, pH 6.5
[0140] Elution buffer: 20mM sodium citrate, 0.2M NaCl, 0.5M arginine hydrochloride, 0.02% Polysorbate80, pH 6.5
[0141] experiment settings
[0142] The column was equilibrated with equilibratio...
Embodiment 3
[0150] raw material
[0151] Recombinant human FIX (rhFIX) was produced in HEK 293 cells. Cells are removed and the cell-free supernatant is the starting material for loading onto a Capto MMC column.
[0152] Chromatography Resins and Columns
[0153] The mixed-mode resin Capto MMC (cat number 17-5317) from GE Healthcare was used as the capture step for the FIX molecules. Capto MMC is a weak cationic resin with hydrophobic and thiophilic interactions and hydrogen bonding. The XK26 column was packed with Capto MMC resin to a bed height of 15 cm. The column volume (CV) was 80ml.
[0154] buffer
[0155] Equilibration buffer: 20mM sodium citrate, 0.1M NaCl, pH 7.0
[0156] High Salt Wash Buffer: 20mM Sodium Citrate, 0.7M NaCl, pH 6.5
[0157] Elution buffer: 20mM sodium citrate, 0.2M NaCl, 0.8M arginine hydrochloride, pH 6.5 Experimental setup and results
[0158] The column was equilibrated with equilibration buffer and then loaded with material at a flow rate of 26 ml / m...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com