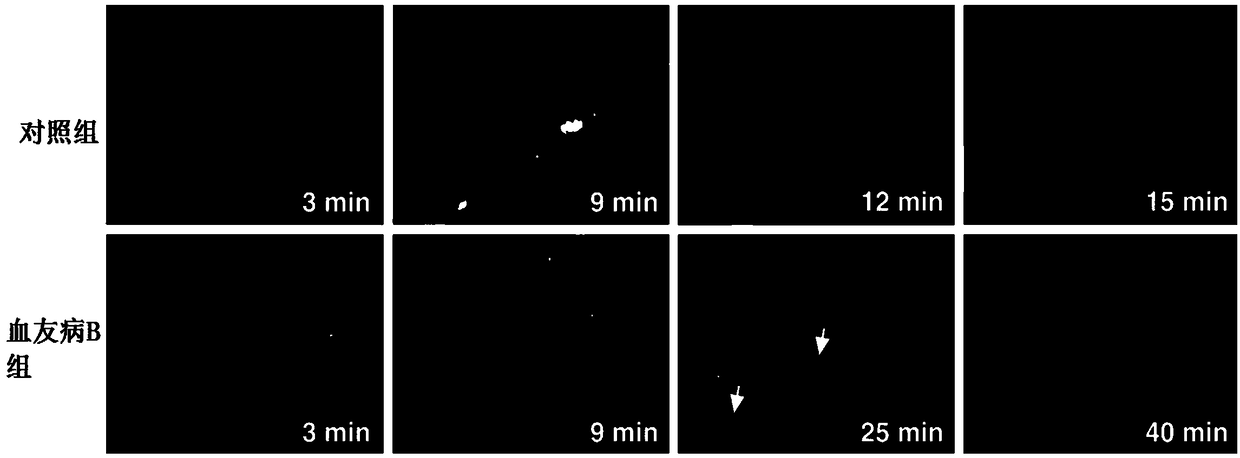
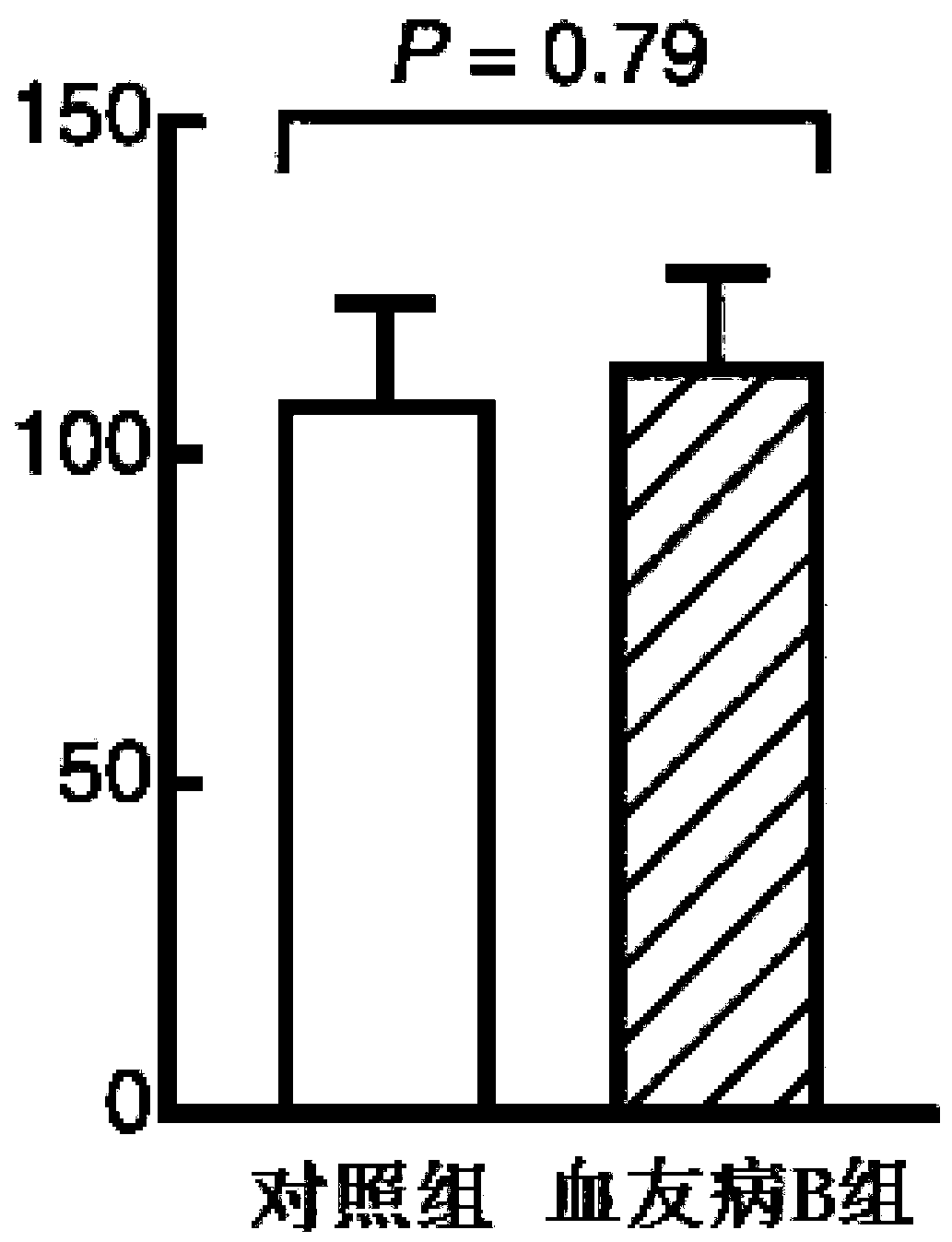
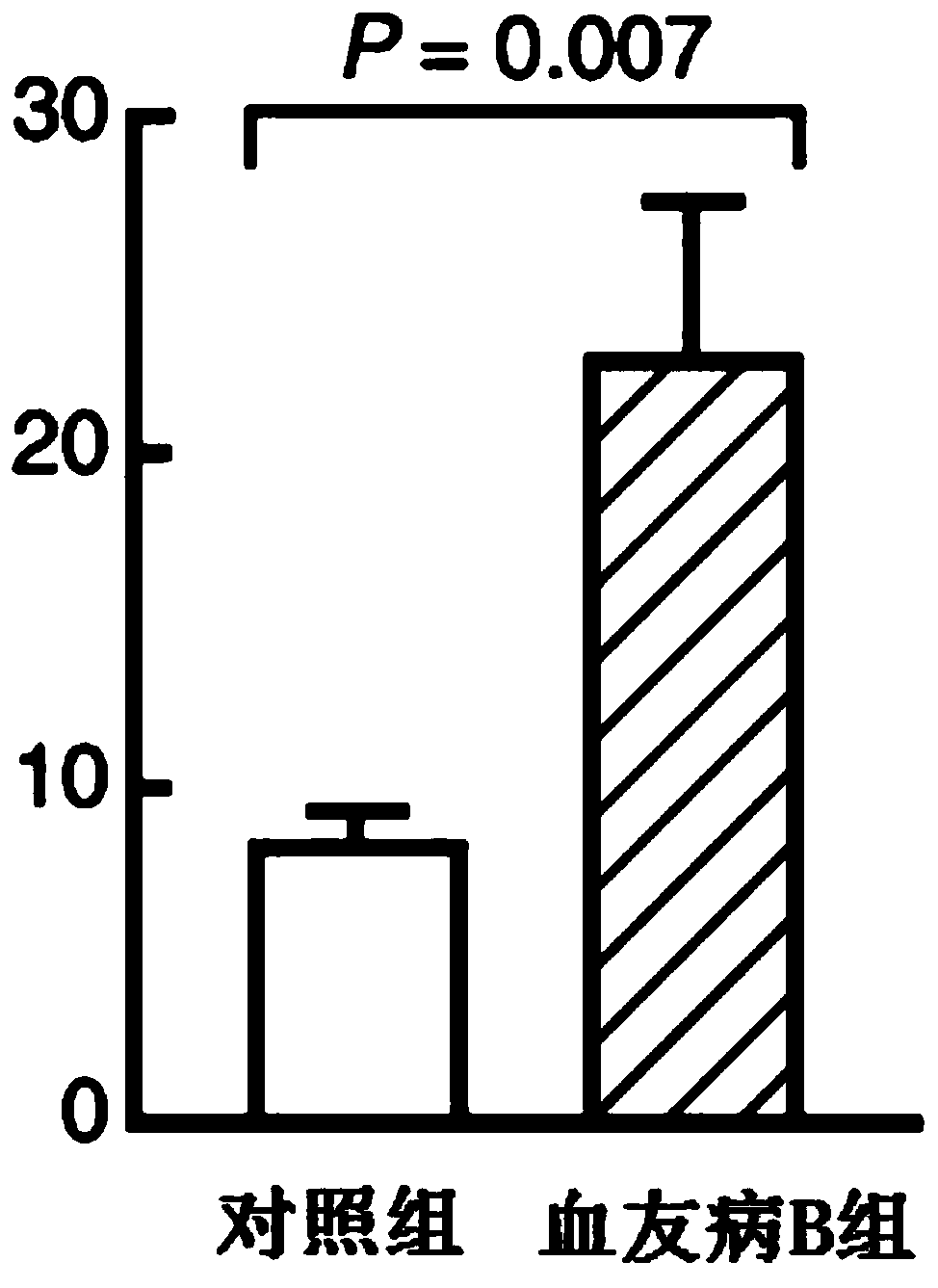
Pharmaceutical composition of hemocoagulase and application of pharmaceutical composition
The technology of a composition and hemagglutinase is applied in the field of pharmaceutical composition of snake venom hemagglutinase, which can solve the problem of lack of radical cure for hemophilia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Freeze-dried powder formulation
[0045] Snake Venom Hemagglutinin 2.5mg
[0046] Glycine 2g
[0047] Mannitol 10g
[0048] Preparation:
[0049] 1. Weigh 10g of mannitol, add 300ml of water for injection, heat and stir to dissolve completely;
[0050] 2. Weigh 2g of glycine, add it to the above solution, stir to dissolve completely;
[0051] 3. After the solution is allowed to cool, add water for injection to make up to 500ml, and filter with a 0.22μm filter membrane;
[0052] 4. After sterile filtration, add 2.5 mg of snake venom hemocoagulase (the mass ratio of coagulation factor X activator and batroxobin is 1:1), mix well, quantitatively fill 0.5ml / cartridge, and fill a total of 1000 Put the half-pressed rubber stopper into the freeze dryer for freeze-drying;
[0053] 5. Freeze-drying parameters: Pre-freezing temperature is preferably -30°C, pre-freezing time is 2 hours, freeze-drying temperature is -20°C, freeze-drying time is 6 hours, secondary freeze-dryin...
Embodiment 2
[0055] Freeze-dried powder formulation
[0056] Snake venom hemagglutinin 0.25mg
[0057] Glycine 8g
[0058] Mannitol 60g
[0059] Preparation:
[0060] 1. Weigh 60g of mannitol, add 300ml of water for injection, heat and stir to dissolve completely;
[0061] 2. Weigh 8g of glycine, add it to the above solution, stir to dissolve completely;
[0062] 3. After the solution is allowed to cool, add water for injection to make up to 500ml, and filter with a 0.22μm filter membrane;
[0063] 4. After sterile filtration, add 0.25 mg of snake venom hemagglutinin (the mass ratio of blood coagulation factor X activator and snake venom batroxobin is 1:8), mix well, quantitatively fill 0.5ml / cartridge, and fill together 1000 pieces, half-pressed rubber stoppers are placed in a freeze dryer for freeze-drying;
[0064] 5. Freeze-drying parameters: Pre-freezing temperature is preferably -30°C, pre-freezing time is 2 hours, freeze-drying temperature is -20°C, freeze-drying time is 6 hou...
Embodiment 3
[0066] Freeze-dried powder formulation
[0067] Snake Venom Hemagglutinin 1.05mg
[0068] Glycine 6g
[0069] Mannitol 15g
[0070] Preparation:
[0071] 1. Weigh 15g of mannitol, add 300ml of water for injection, heat and stir to dissolve completely;
[0072] 2. Weigh 6g of glycine, add it to the above solution, stir to dissolve completely;
[0073] 3. After the solution is allowed to cool, add water for injection to make up to 500ml, and filter with a 0.22μm filter membrane;
[0074] 4. After sterile filtration, add 1.05 mg of snake venom hemocoagulase (the mass ratio of blood coagulation factor X activator and Agkistrodon brasiliensis batroxobin is 1:5), mix evenly, quantitatively fill 0.5ml / cartridge, total Fill 1000 pieces, put the semi-pressed rubber stopper into the freeze dryer for freeze drying;
[0075] 5. Freeze-drying parameters: Pre-freezing temperature is preferably -30°C, pre-freezing time is 2 hours, freeze-drying temperature is -20°C, freeze-drying time ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com