Preparation method of high-purity human coagulation factor IX
A technology of human blood coagulation factor and blood coagulation factor, which is applied in the field of biomedicine, can solve problems such as high cost, low product safety, and complicated process, and achieve the effects of less loss, reduced probability of thrombin activation, and simple process operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
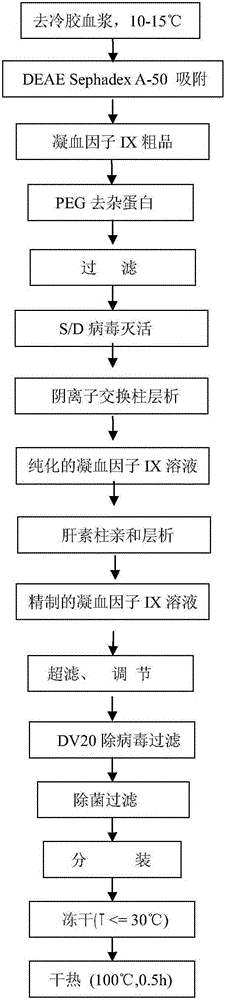
[0025] A method for preparing high-purity human coagulation factor IX includes the following steps:
[0026] 1) Take 35 bags of frozen plasma, rinse the surface with 70% ethanol and rinse with cold water for injection, cut the bags, pour them into a stainless steel melting slurry bucket, melt in a 0-3℃ water bath, then accurately weigh 20kg and remove by centrifugation Cryoprecipitate to obtain 19.8 kg of cold-gelatinized plasma, and then heat the cold-gelatinized plasma to 13-15°C in a 15°C water bath;
[0027] 2) Weigh 20 grams of DEAE-Sephadex A-50 gel and place it in a gel bucket, first fully swell it with about 2kg of hot water for injection (about 80°C), and then rinse and cool it with about 2kg of cold water for injection (about 15°C). Then equilibrate with about 1kg buffer (PH6.50, 0.01MNa-citrate, 0.075MNaCL, 15℃), then add it to the cold glue-free plasma, stir slowly for 1 hour, and then stand for 10 minutes;
[0028] 3) Filter the cold glue plasma with filter cloth in the...
Embodiment 2
[0040] A preparation method of high-purity human coagulation factor FIX includes the following steps:
[0041] 1) Same as Example 1;
[0042] 2) The dosage, swelling and cooling of DEAE-Sephadex A-50 gel are the same as those in Example 1, and then equilibrate with about 1kg buffer (PH7.35, 0.01MNa-citrate, 0.15MNaCL, 15℃), and then add to the decold gel In the plasma, stir slowly for 1 hour and then stand for 20 minutes;
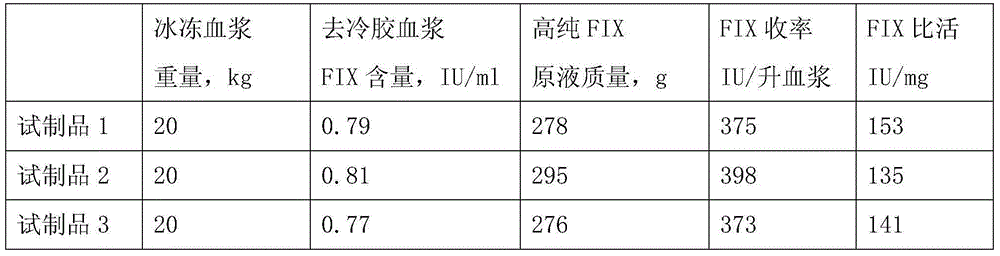
[0043] 3) Filter the cold glue plasma with filter cloth in the gel bucket, collect DEAE-SephadexA-50 gel, and then add 2 liters of washing buffer (PH7.35, 0.015MNa-citrate, 0.2MNaCL) to the gel bucket Stir for about 5 minutes, after filtering through a filter cloth, drain the washing solution from the bottom of the bucket, and repeatedly wash the gel 6 times; then wash with elution buffer (PH7.35, 0.015MNa-citrate, 0.02MArginineHydrochloride, 1.0MNaCL) Degelling 4 times, 1 liter each time, collect about 3988 g of the eluate, filter the eluate with a 0.45 μm filt...
Embodiment 3
[0055] A method for preparing high-purity human coagulation factor IX includes the following steps:
[0056] 1) Same as Example 1;
[0057] 2) The dosage, swelling and cooling of DEAE-Sephadex A-50 gel are the same as in Example 1, and then equilibrate with about 1kg buffer (PH7.05, 0.01MNa-citrate, 0.10MNaCL, 15℃), and then add to the cold gel In the plasma, stir slowly for 1 hour and then stand for 20 minutes;
[0058] 3) Filter the cold glue plasma with filter cloth in the gel bucket, collect DEAE-SephadexA-50 gel, and then add 2 liters of washing buffer (PH7.05, 0.01MNa-citrate, 0.15MNaCL) to the gel bucket Stir for about 8 minutes, after filtering through a filter cloth, drain the washing solution from the bottom of the bucket, and wash the gel 5 times repeatedly, and then wash with elution buffer (PH7.05, 0.01MNa-citrate, 0.02MArginineHydrochloride, 0.65MNaCL) Degelling 3 times, 1 liter each time, collect about 2955g of the eluate, filter the eluate with a 0.45μm filter eleme...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com