Patents
Literature
66 results about "Blood coagulation disorder" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Condition in which there is a deviation from or interruption of the normal coagulation properties of the blood.
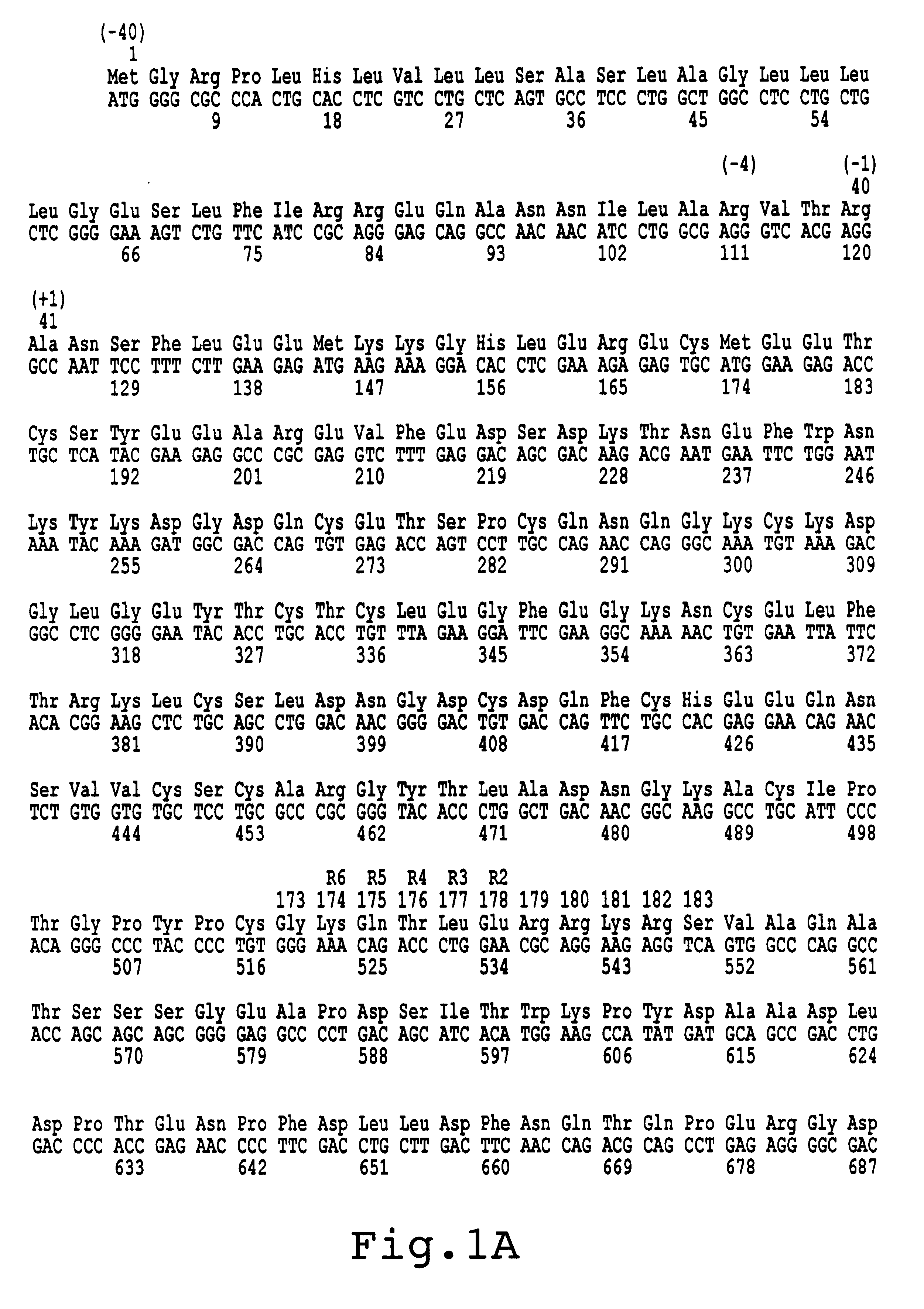
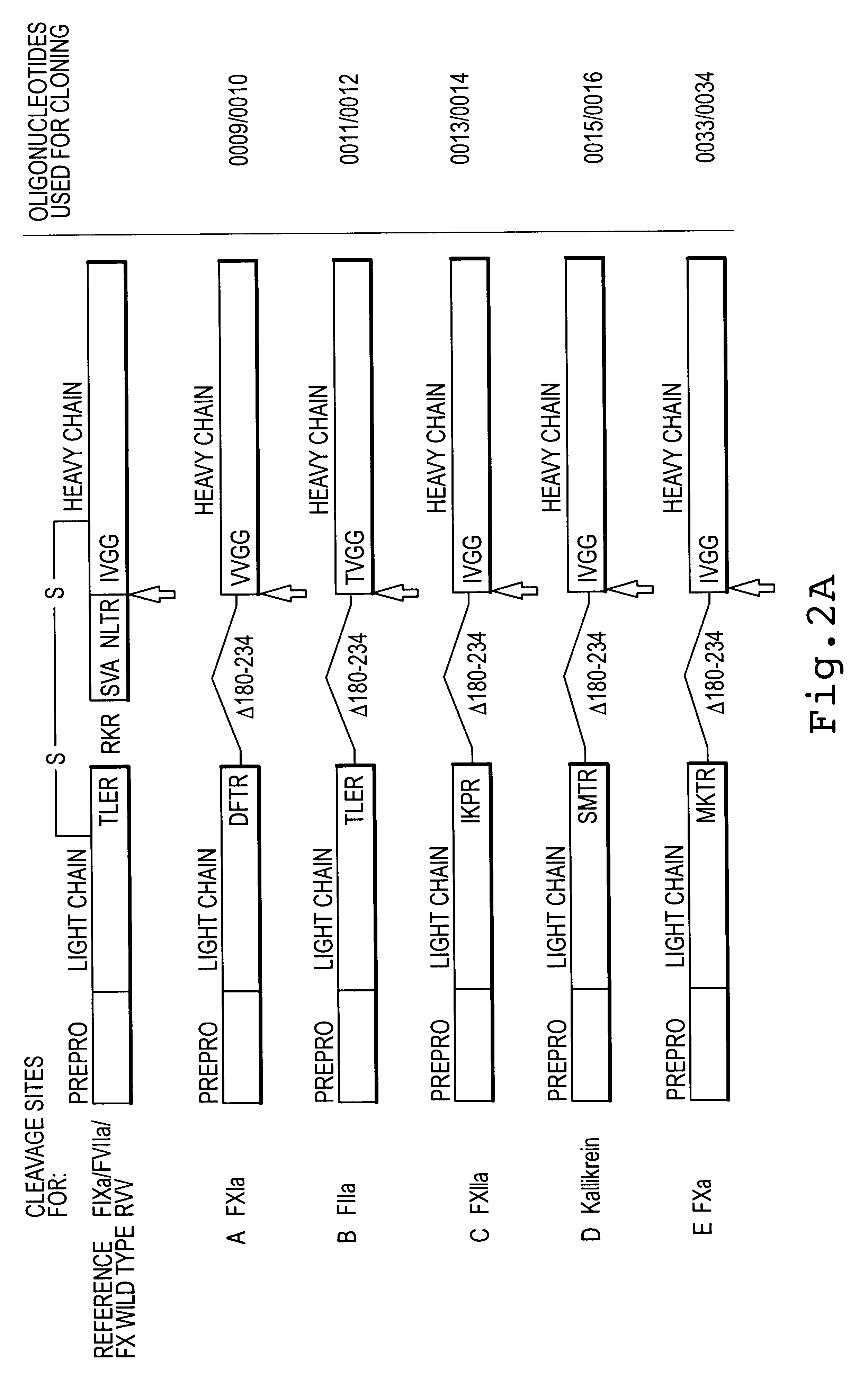
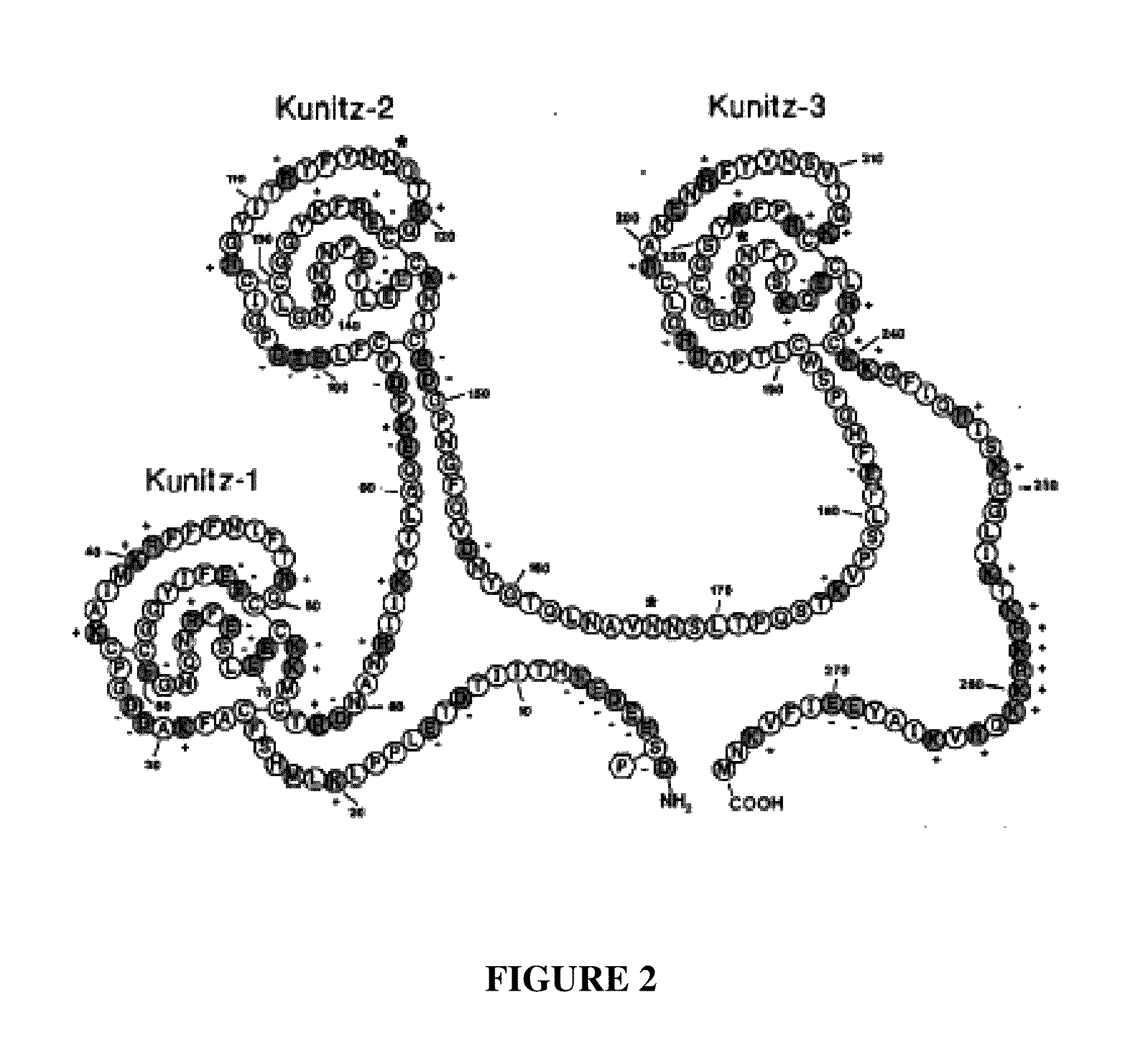
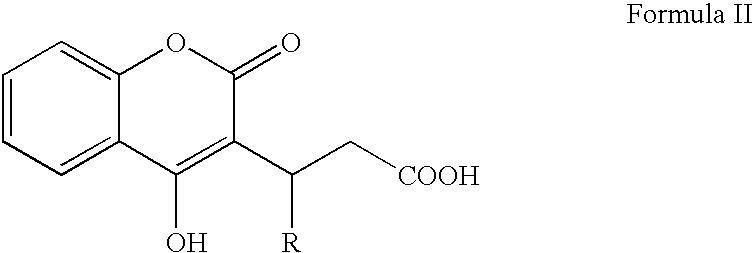
Factor X deletion mutants and analogues thereof
InactiveUS6562598B1Improve stabilityHigh stability and structural integrityFungiBacteriaFactor XAmino acid
Factor XDELTA analogues are provided, as well as pharmaceutical preparations containing such analogues and methods of preparing such analogues. The factor XDELTA analogues have a deletion of the amino acids Arg180 to Arg234 and a modification in the region of the amino acid sequence between Gly173 and Arg179 of the factor X amino acid sequence. Such analogues can include a processing site not normally present in factor X, thus allowing for selective conversion of the analogue to an active form. The analogues and preparations have utility in the treatment of a number of blood coagulation disorders.
Owner:BAXALTA INC
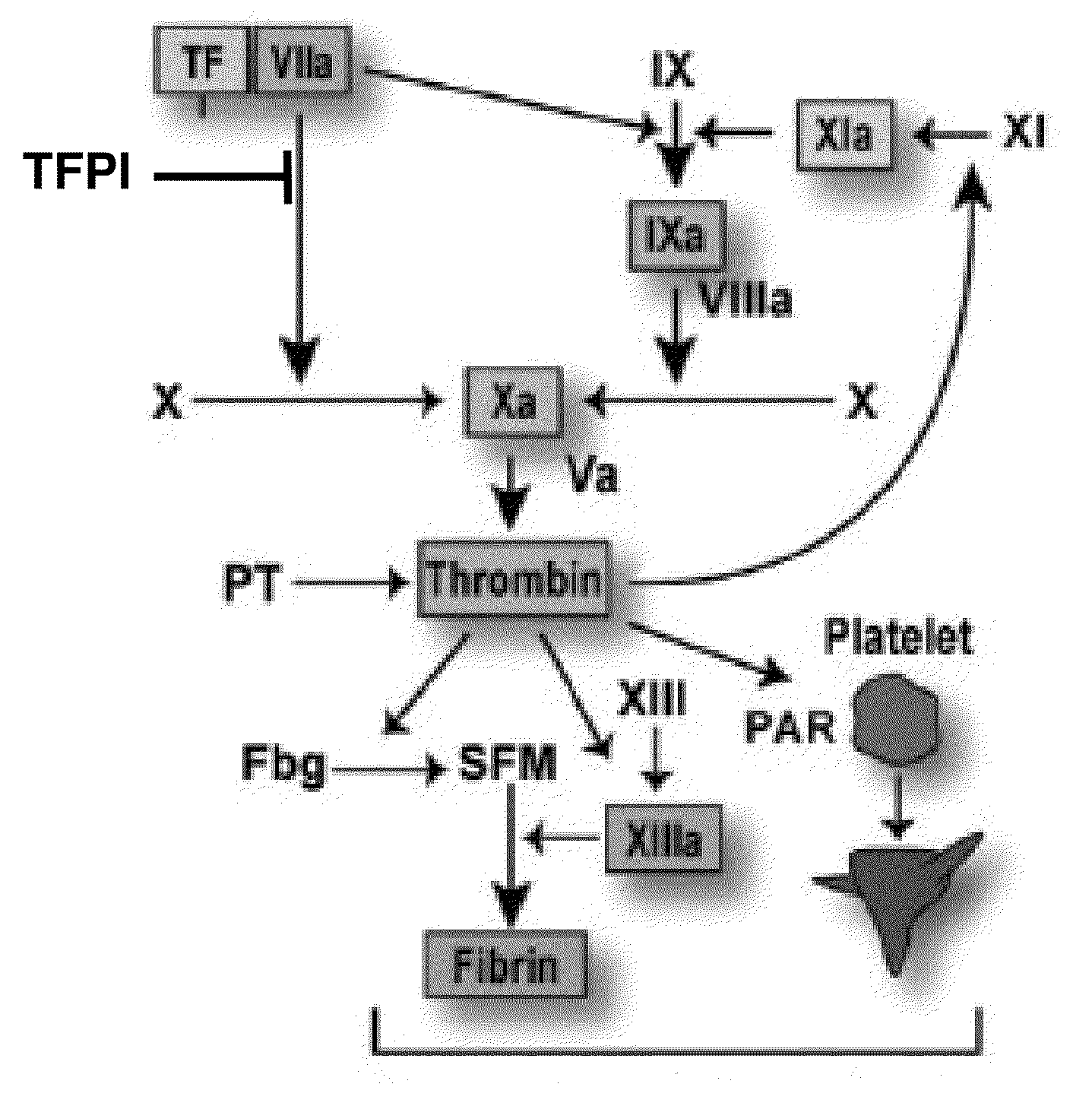
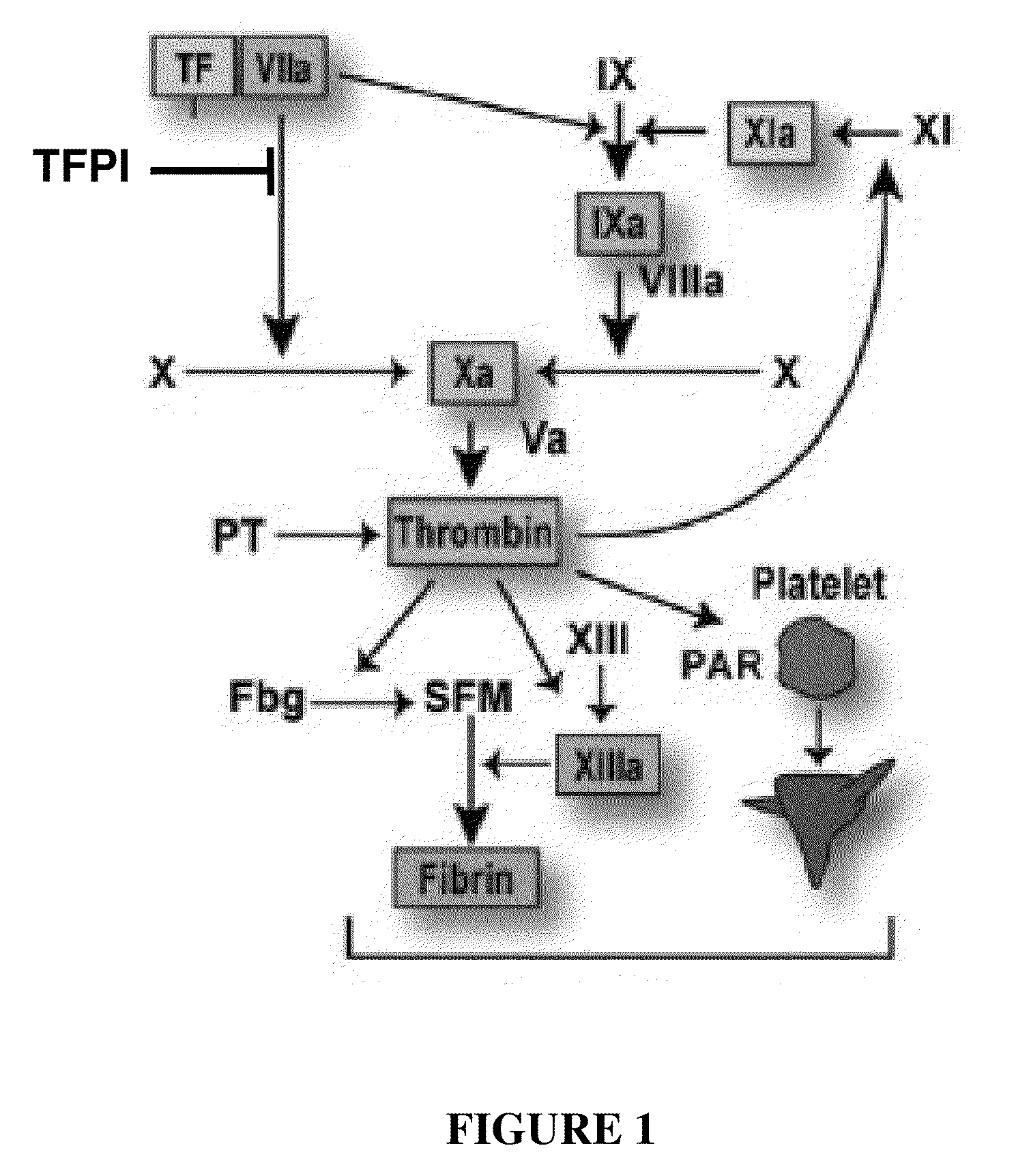
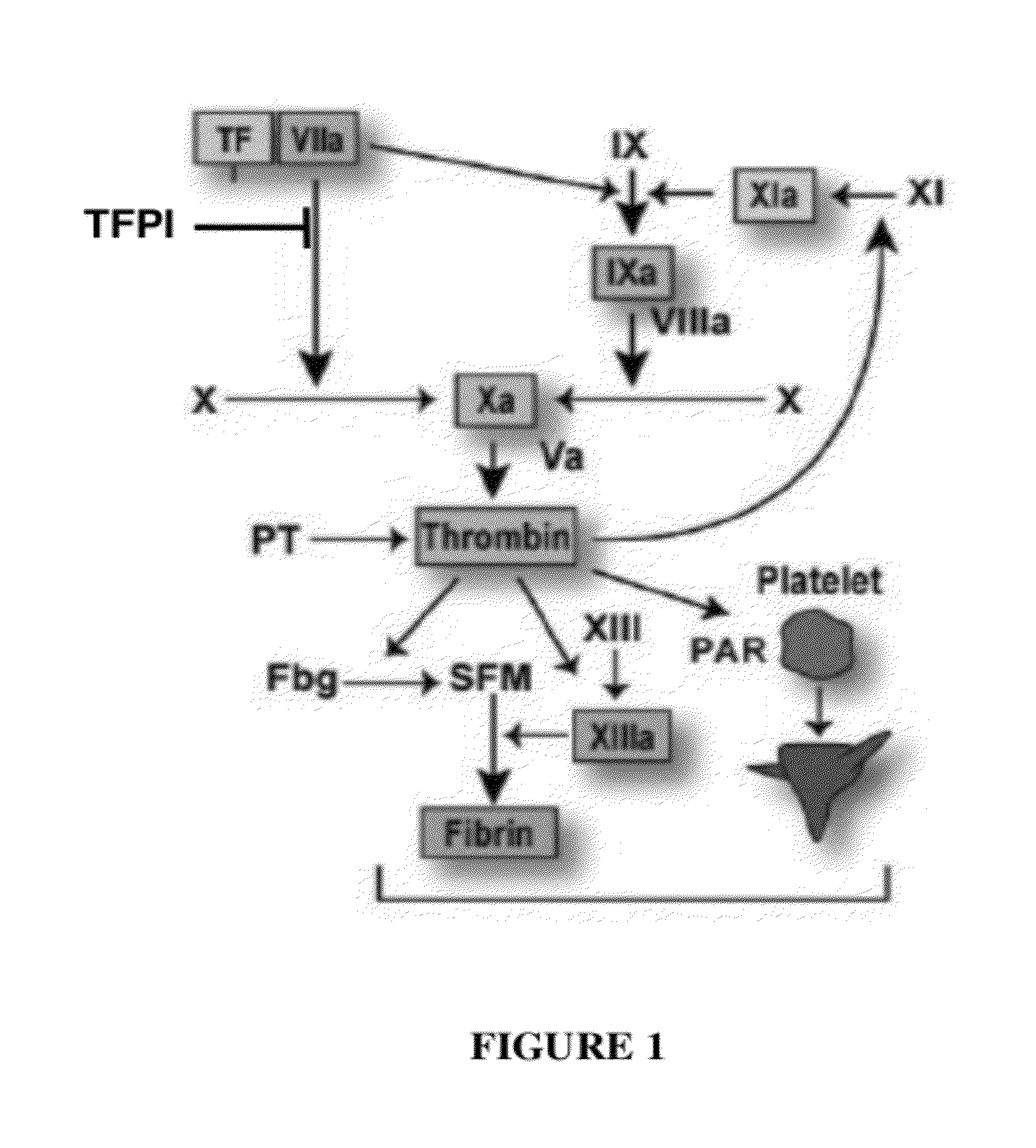
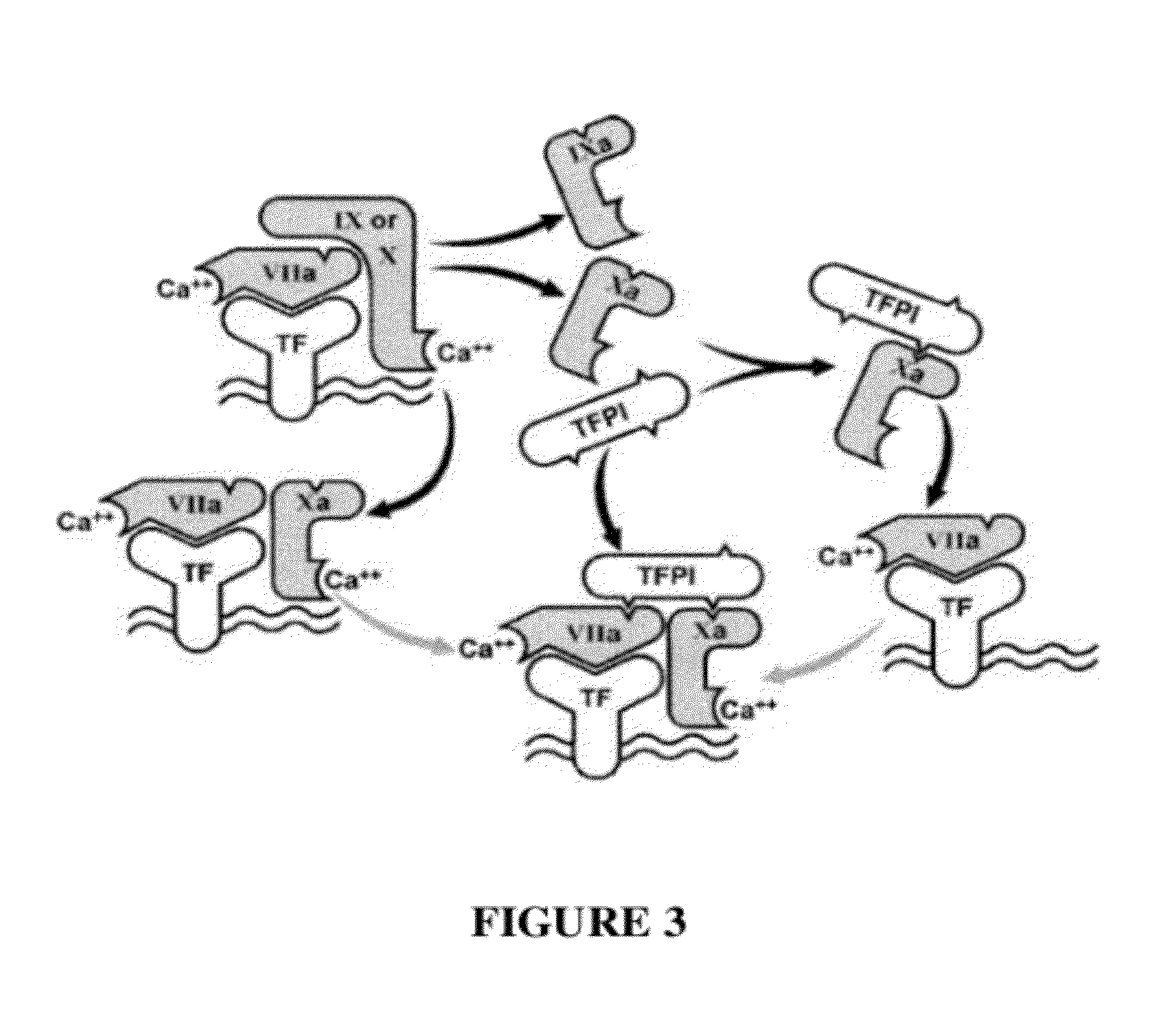
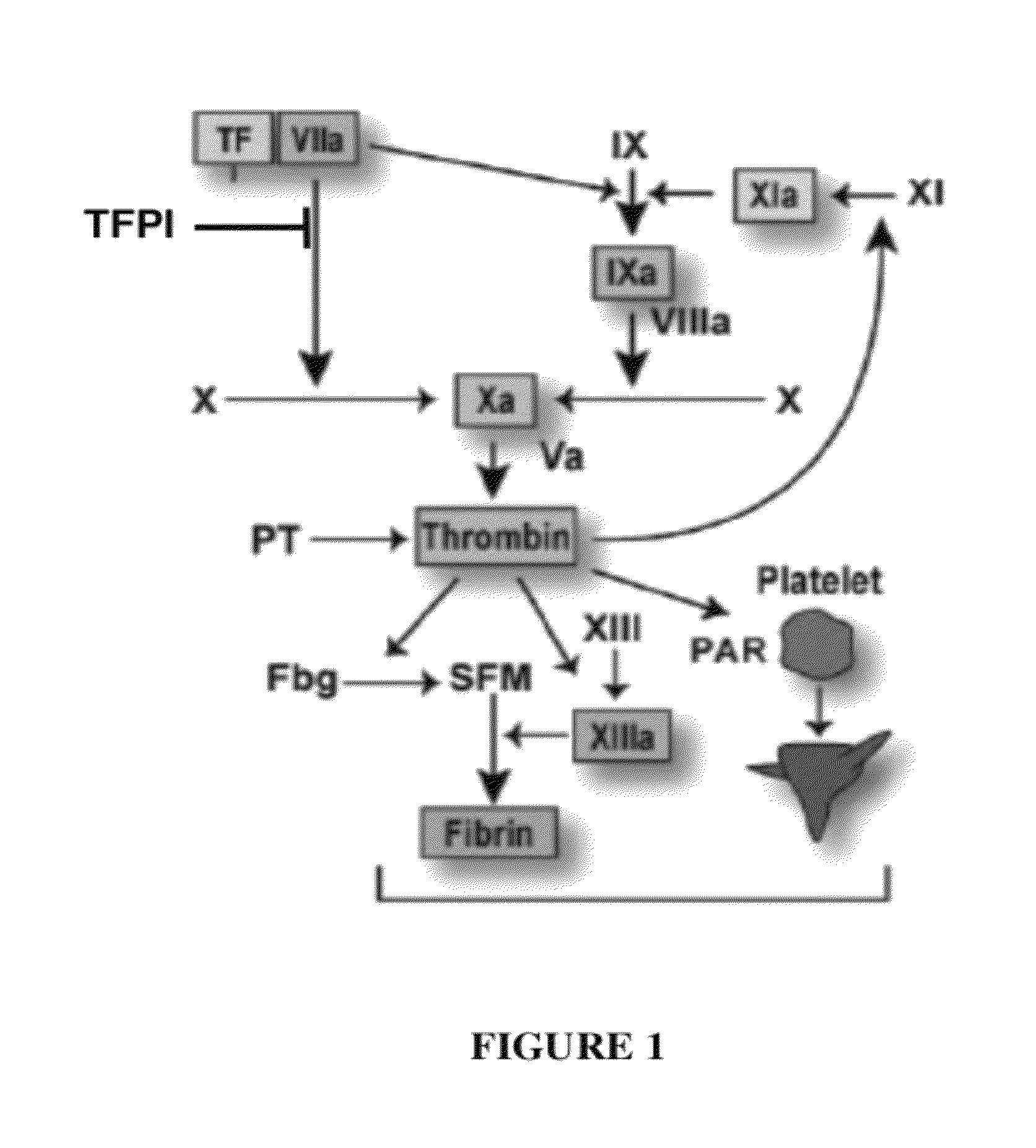
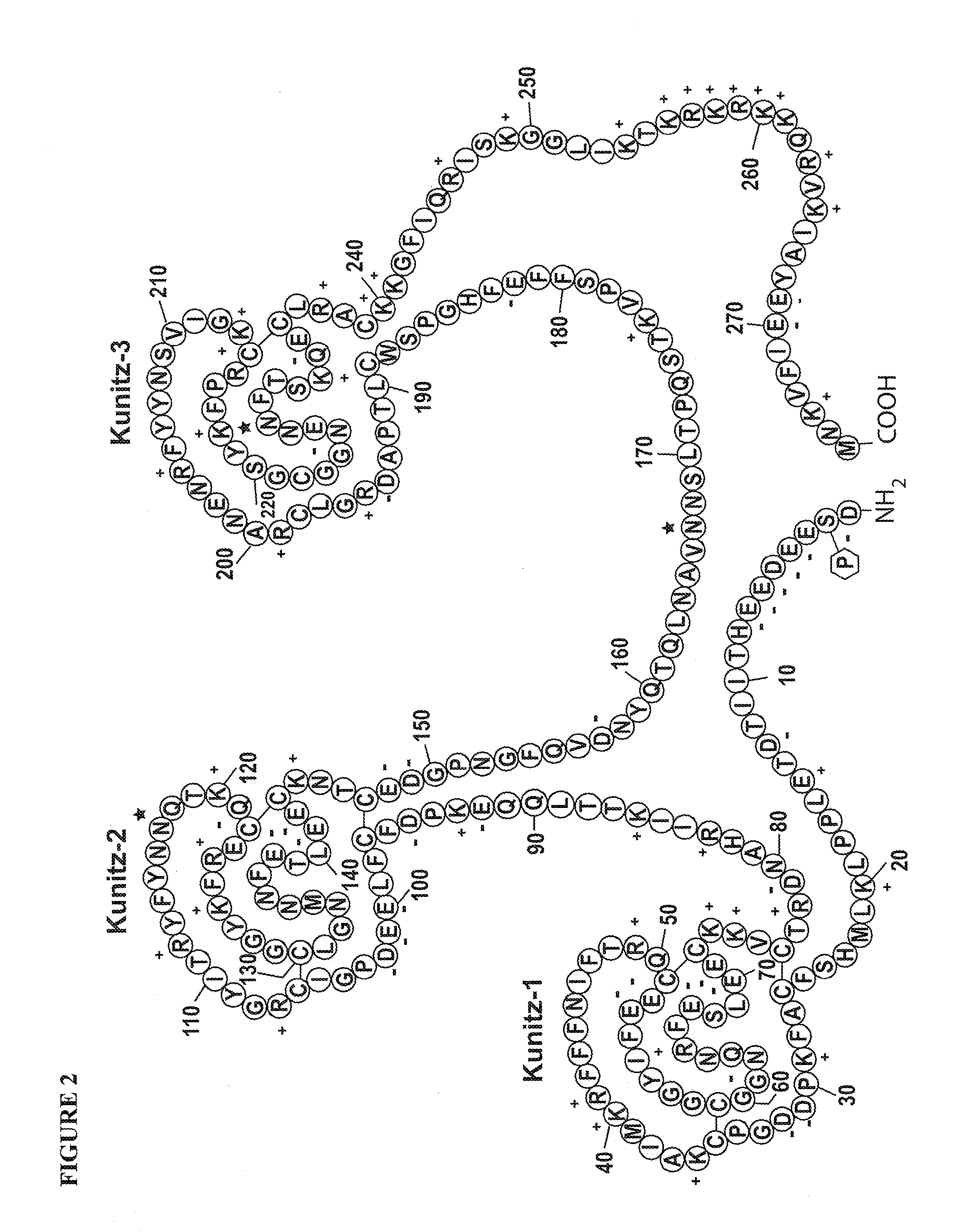
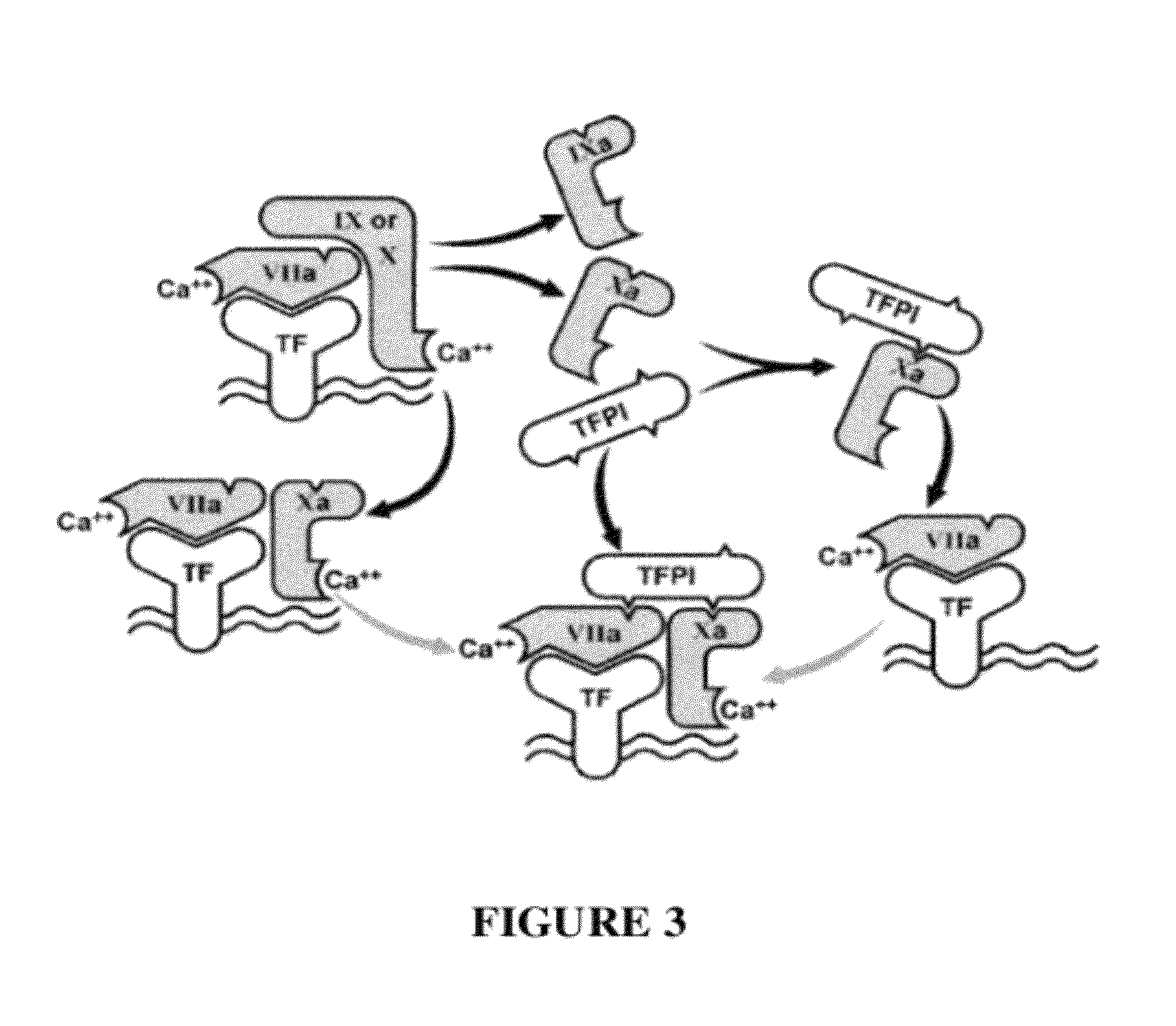
TFPI inhibitors and methods of use
ActiveUS20100173847A1Improves TFPI-regulated thrombin generationFactor VIIPeptide-nucleic acidsMedicineThrombin activity
The invention provides peptides that bind Tissue Factor Pathway Inhibitor (TFPI), including TFPI-inhibitory peptides, and compositions thereof. The peptides may be used to inhibit a TFPI, enhance thrombin formation in a clotting factor-deficient subject, increase blood clot formation in a subject, and / or treat a blood coagulation disorder in a subject.
Owner:TAKEDA PHARMA CO LTD
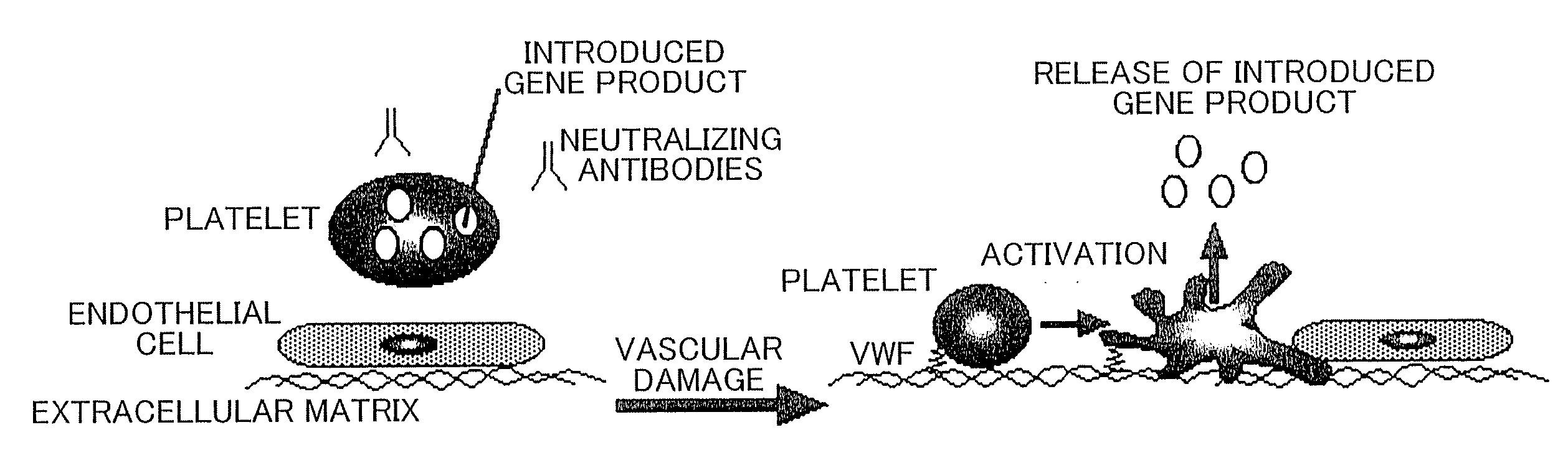
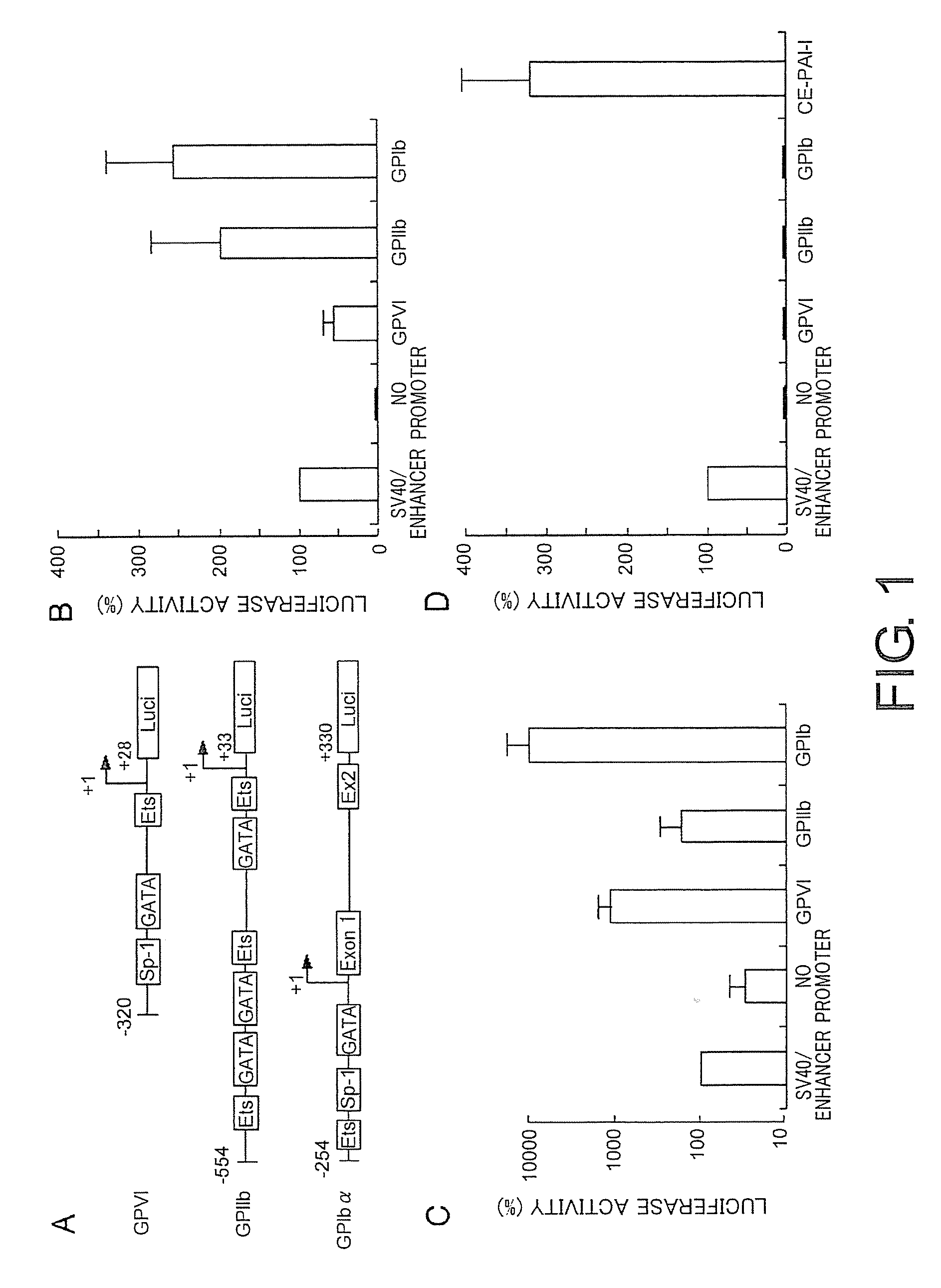
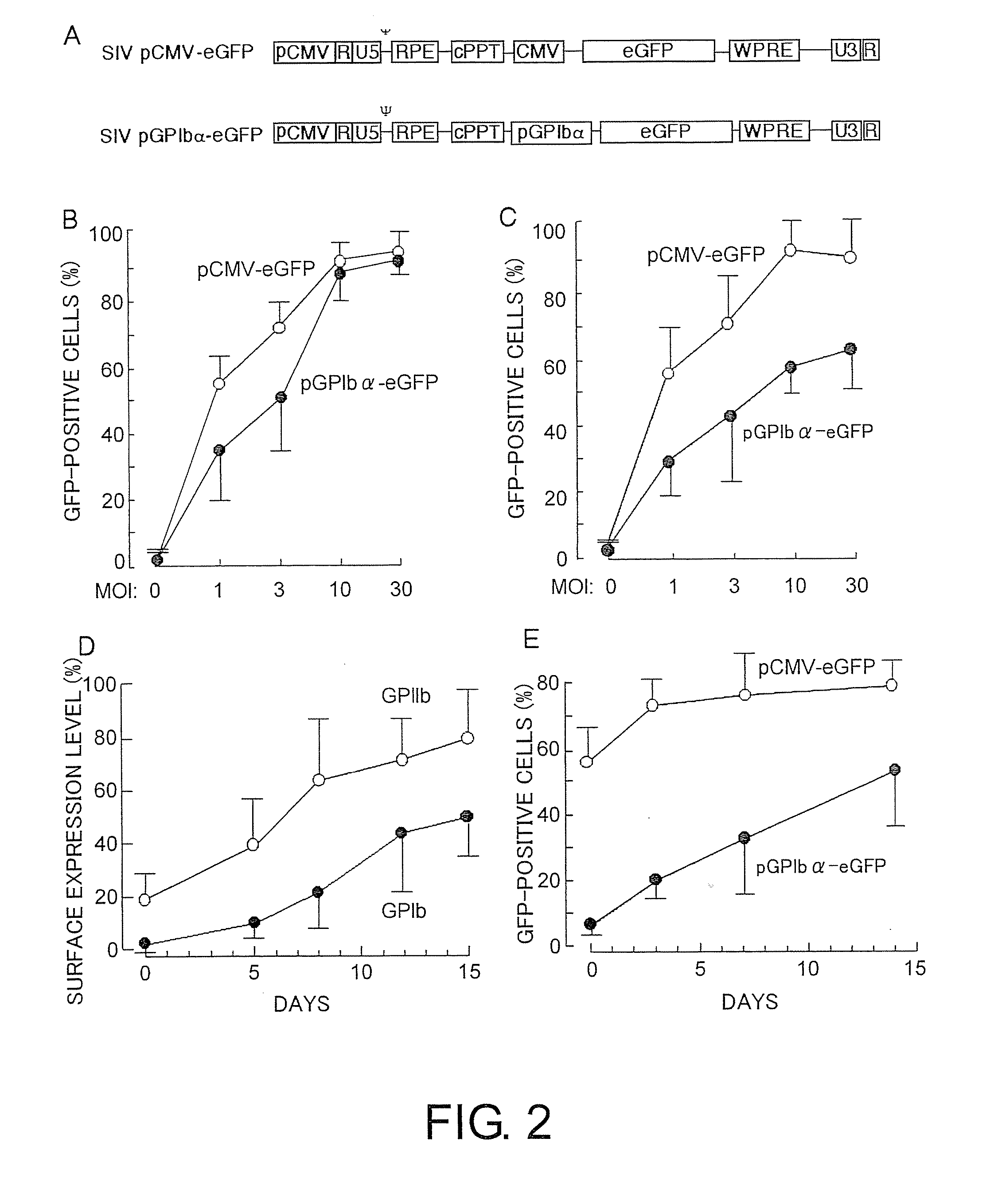
Therapeutic method for blood coagulation disorder
InactiveUS20090148425A1Ensure adequate treatmentImprove abilitiesBiocidePeptide/protein ingredientsFactor iiPlatelet
The present invention provides agents for treating blood coagulation abnormalities, which contain as an active ingredient a lentiviral vector carrying a blood coagulation factor gene operably linked to a promoter which induces platelet-specific expression. Agents for treating hemophilia A or hemophilia B are provided by application of the gene encoding Factor VIII or Factor IX. Blood coagulation abnormalities can be treated by gene therapy by infecting hematopoietic stem cells or such with the therapeutic agents of the present invention.
Owner:DNAVEC CORP
Methods for treating blood coagulation disorders
InactiveUS7615537B2Optimally promote proper disulfide bondingPrecise BondingBiocideVirusesFactor VIIaA-DNA
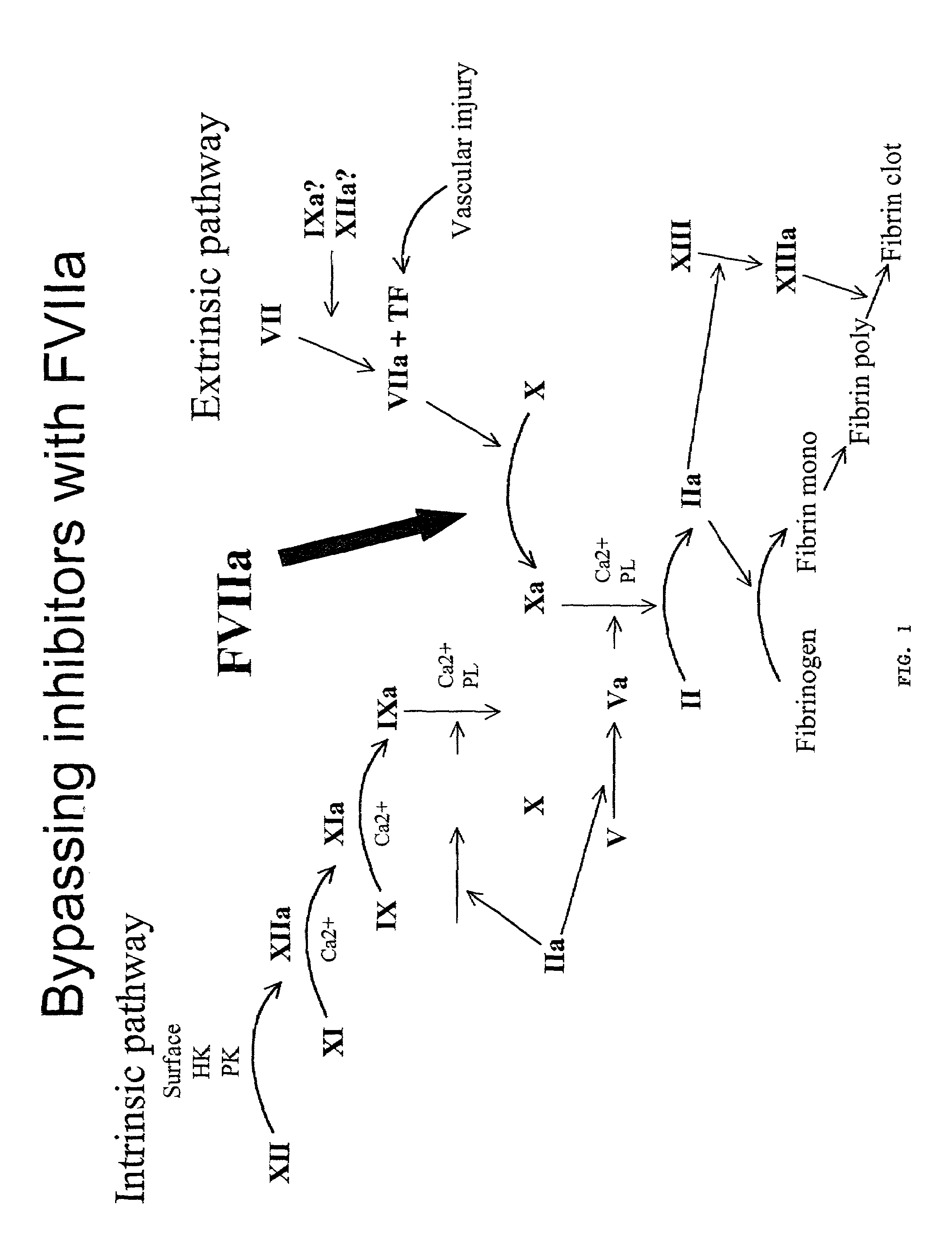
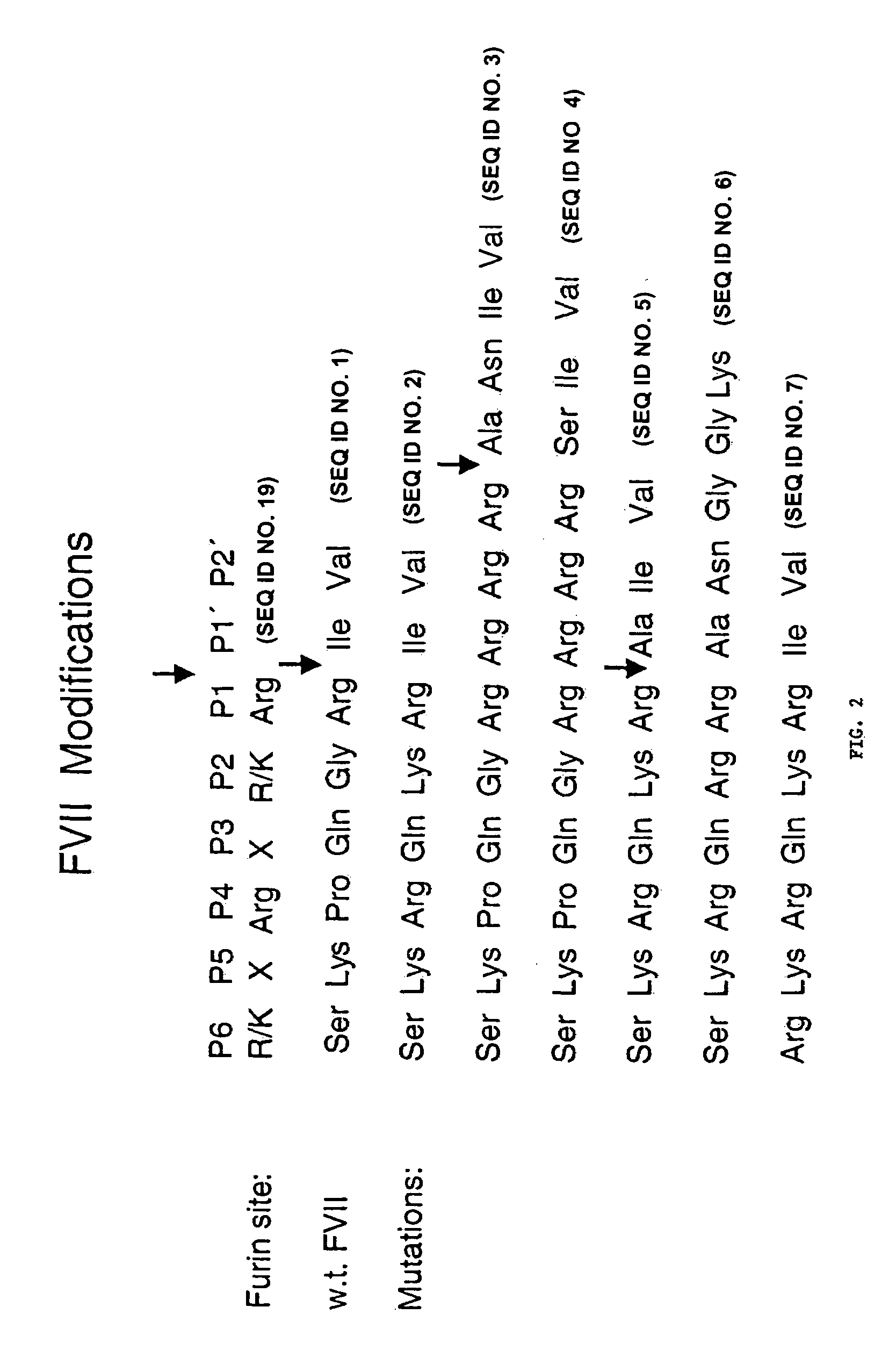
The present invention relates to a method of treating an individual having a blood coagulation defect (e.g., hemophilia A, hemophilia B), comprising administering to the individual an effective amount of a DNA vector encoding modified Factor VII (FVII), wherein the modified Factor VII leads to generation of Factor VIIa in vivo. In a particular embodiment, the invention pertains to a method of treating an individual having a blood coagulation defect comprising administering to the individual an effective amount of a nucleic acid encoding a modified FVII wherein the modified FVII comprises a signal which codes for precursor cleavage by furin at the activation cleavage site of the modified FVII. The invention also relates to a method of treating an individual having a blood coagulation disorder comprising administering to the individual an effective amount of a nucleic acid encoding the light chain of human FVII and a nucleic acid encoding the heavy chain of human FVII operably linked to a leader sequence. Compositions, expression vectors and host cells comprising nucleic acid which encodes a modified Factor VII, wherein the modified Factor VII leads to generation of Factor VIIa in vivo is also encompassed by the present invention.
Owner:GENZYME CORP
TFPI inhibitors and methods of use
ActiveUS20120028901A1Improves TFPI-regulated thrombin generationIncrease the number ofPeptide/protein ingredientsLibrary screeningMedicineThrombin activity
The invention provides peptides that bind Tissue Factor Pathway Inhibitor (TFPI), including TFPI-inhibitory peptides, and compositions thereof. The peptides may be used to inhibit a TFPI, enhance thrombin formation in a clotting factor-deficient subject, increase blood clot formation in a subject, treat a blood coagulation disorder in a subject, purify TFPI, and identify a TFPI-binding compound.
Owner:TAKEDA PHARMA CO LTD
TFPI inhibitors and methods of use
ActiveUS8450275B2Improves TFPI-regulated thrombin generationIncrease the number ofCompound screeningApoptosis detectionClot formationTissue factor pathway inhibitor
The invention provides peptides that bind Tissue Factor Pathway Inhibitor (TFPI), including TFPI-inhibitory peptides, and compositions thereof. The peptides may be used to inhibit a TFPI, enhance thrombin formation in a clotting factor-deficient subject, increase blood clot formation in a subject, treat a blood coagulation disorder in a subject, purify TFPI, and identify a TFPI-binding compound.
Owner:TAKEDA PHARMA CO LTD
Compositions and methods to control bleeding
InactiveUS20030050225A1Reduce needReducing cost and efficaciousnessFactor VIIPeptide/protein ingredientsMammalBlood coagulations
Disclosed are compositions for treating blood coagulation disorders and allows for manipulation of the blood coagulation cascade. More particularly the invention, relates to compositions for altering bleeding that include a mixture of at least one blood coagulation factor in a low dose and phospholipid vesicles. The invention has a variety of important uses including controlling bleeding in a mammal that has or is suspected of having a potentially life-threatening blood coagulation disorder.
Owner:UNIVERSITY OF VERMONT
Method for treating blood coagulation disorders
Provided is a method for treatment of blood coagulation disorders. The method comprises administering to an individual compositions comprising lipidic particles comprising phosphatidylcholine, phosphatidylinositol and cholesterol. One or more peptides, polypeptides or proteins involved in the blood coagulation cascade are associated with the lipidic particles.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Anticoagulation Agent and Uses Thereof
InactiveUS20090148383A1High affinity bindingTherapy is simplePeptide/protein ingredientsAntibody mimetics/scaffoldsAnticoagulantCoagulation Disorder
The present invention provides an anticoagulant agent including a first element capable of inhibiting coagulation and a second element capable of targeting an activated platelet wherein upon administration of element directs the first element to the activated platelet. Also provided is a probe for detecting a blood vessel abnormality including (a) a binding element capable of targeting an activated platelet and (b) a label. Applicant has shown that agents and probes directed to activated platelets are useful in the diagnosis and therapy of coagulation disorders.
Owner:BAKER IDI HEART & DIABETES INST HLDG LTD
TFPI inhibitors and methods of use
ActiveUS8466108B2Improves TFPI-regulated thrombin generationPeptide-nucleic acidsFactor VIIMedicineThrombin activity
The invention provides peptides that bind Tissue Factor Pathway Inhibitor (TFPI), including TFPI-inhibitory peptides, and compositions thereof. The peptides may be used to inhibit a TFPI, enhance thrombin formation in a clotting factor-deficient subject, increase blood clot formation in a subject, and / or treat a blood coagulation disorder in a subject.
Owner:TAKEDA PHARMA CO LTD
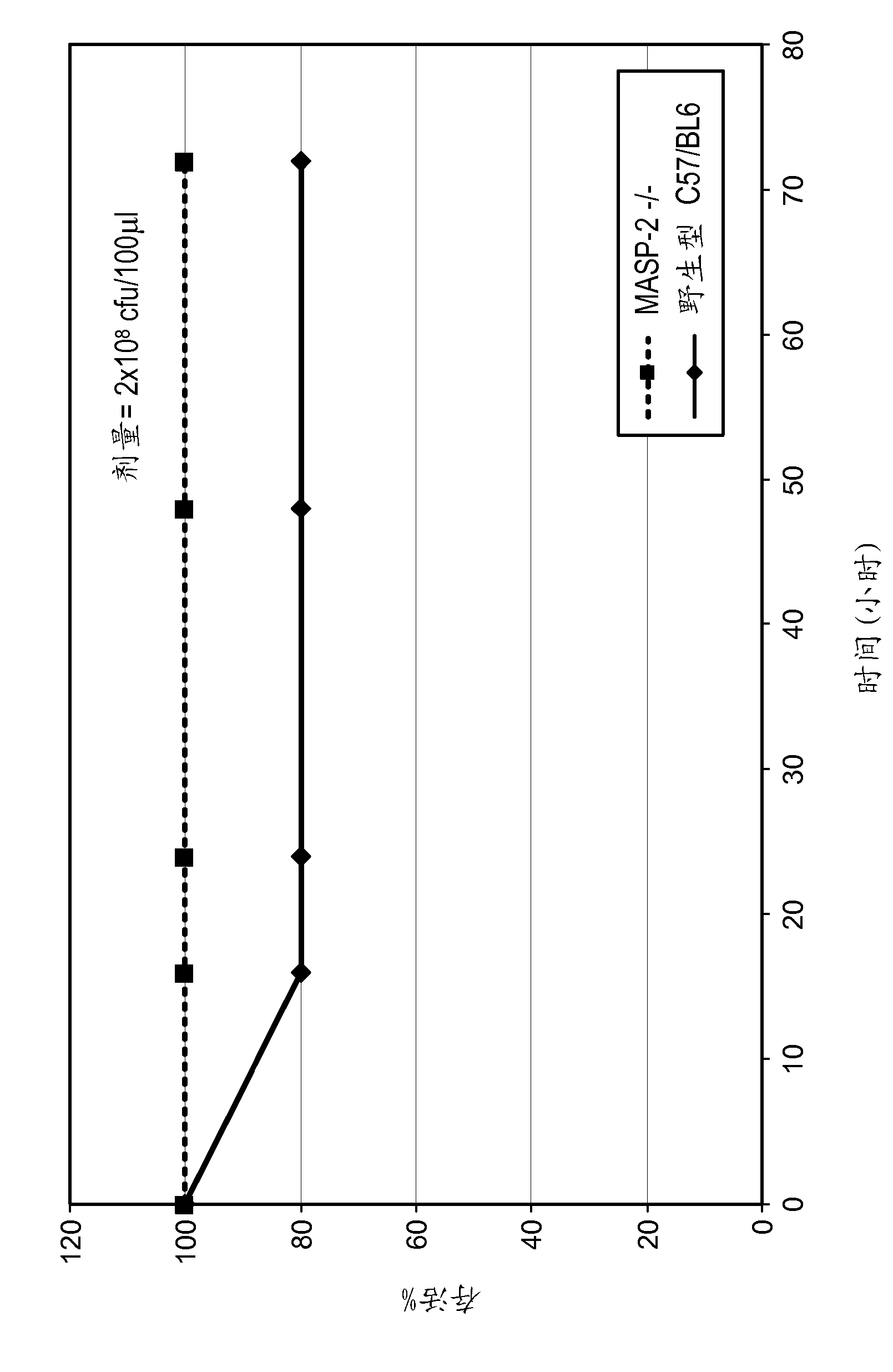
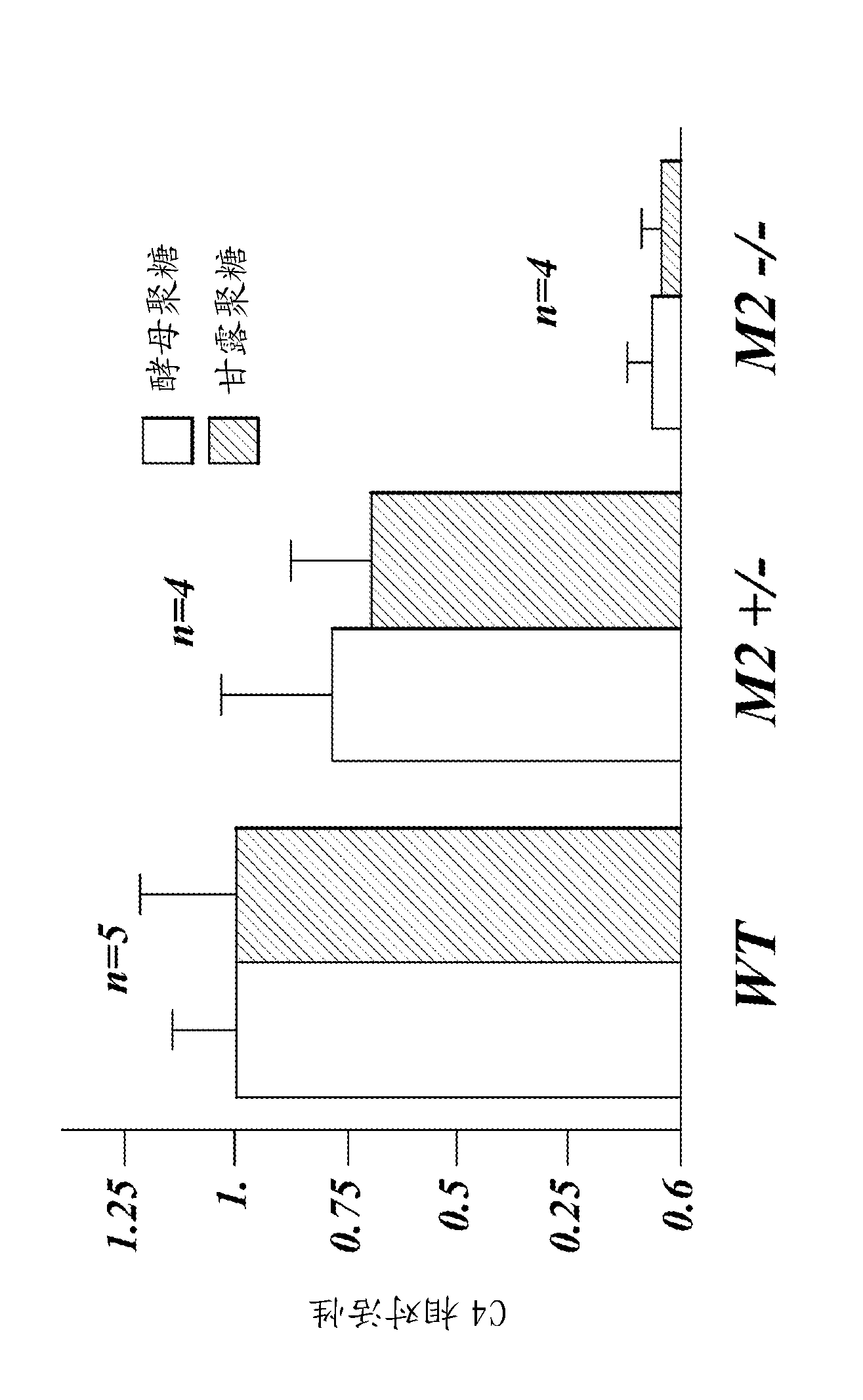
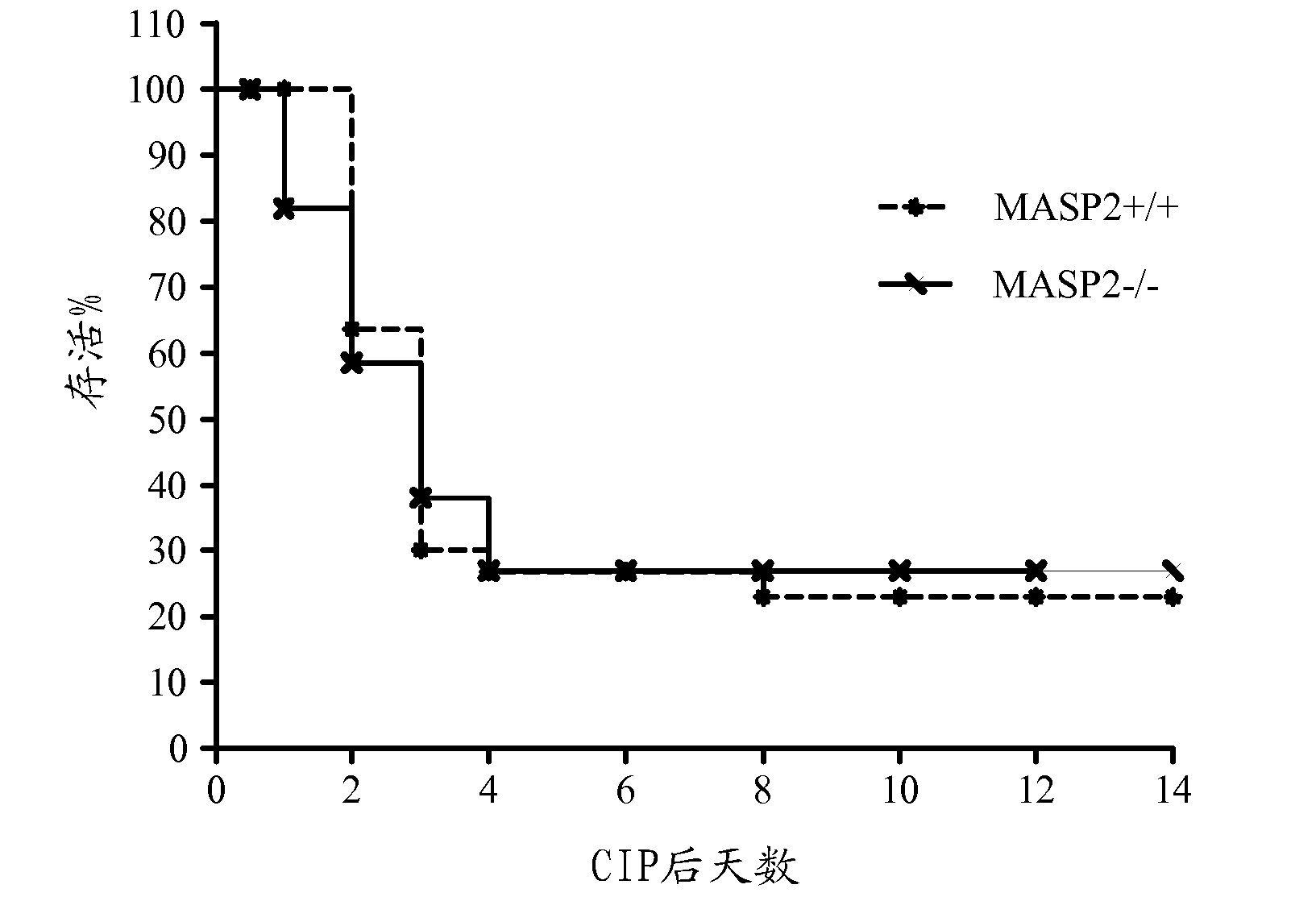
Methods for treating disseminated intravascular coagulation by inhibiting MASP-2 dependent complement activation
ActiveCN102781471AReduce severityAntibacterial agentsNervous disorderDisseminated coagulopathyPharmaceutical medicine
Owner:OMEROS CORP +1
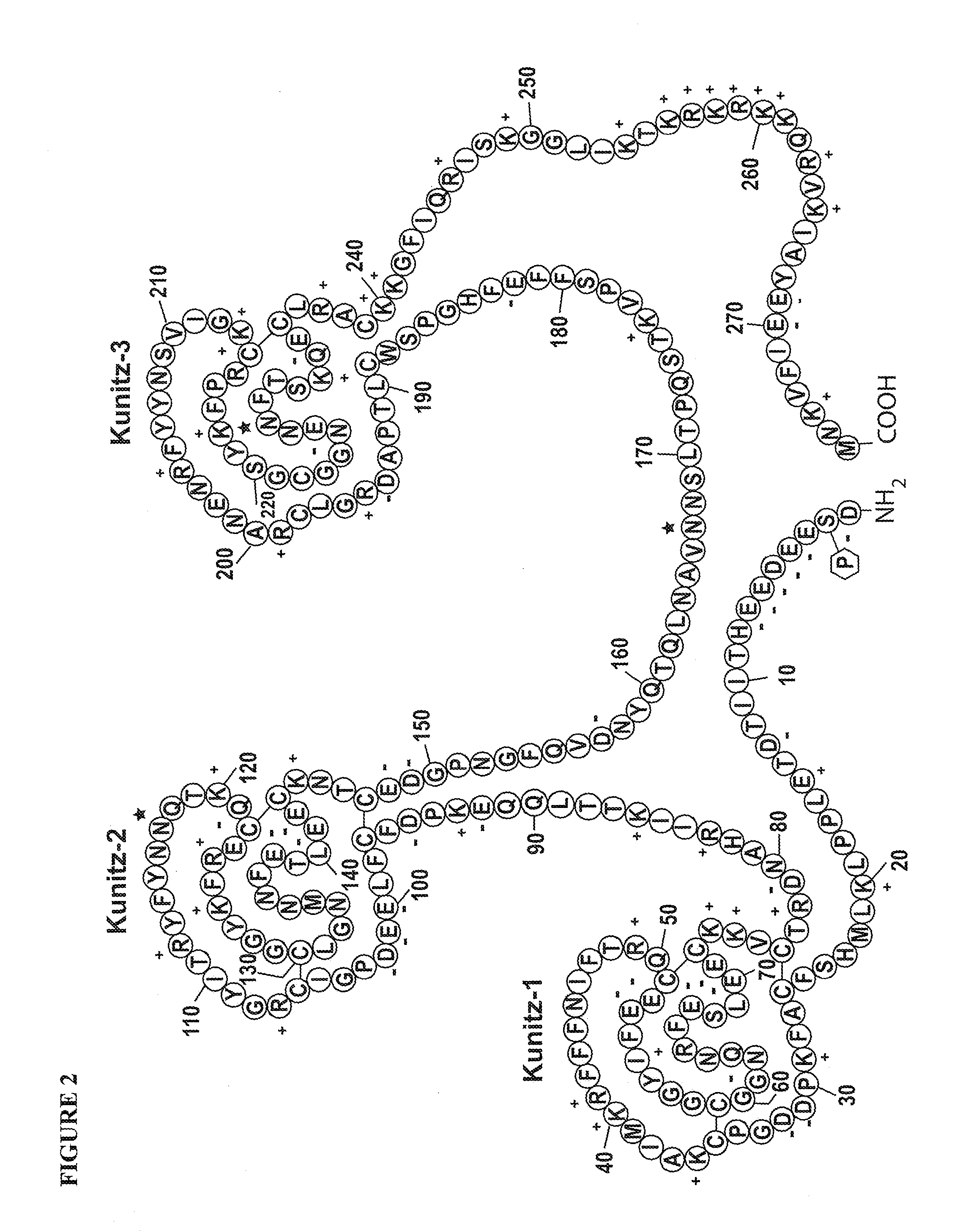
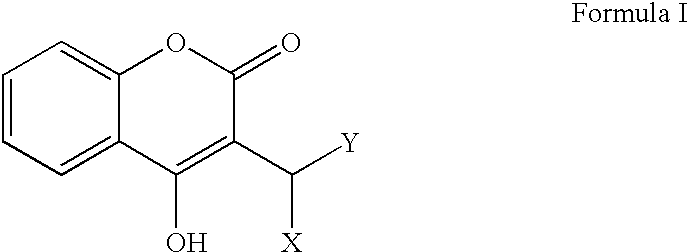
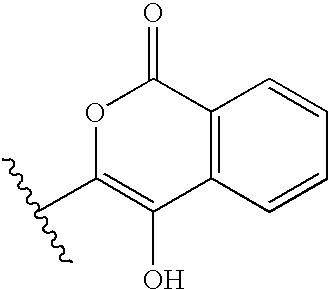
Serine protease derivatives and uses in the prevention or the treatment of blood coagulation disorders
ActiveUS8436144B2Extended half-lifePromote recoveryPeptide/protein ingredientsAntibody mimetics/scaffoldsZymogenWAS PROTEIN
The present invention relates to chimeric derivatives of serine protease zymogen containing the activation peptide of factor X or a fragment thereof for improving the half-life of said derivatives. Preferably, said chimeric derivatives are protein C and factor X derivatives. The invention also relates to said derivatives for the prevention or treatment of blood coagulation disorders.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
Serine protease derivatives and uses in the prevention or the treatment of blood coagulation disorders
ActiveUS20110293597A1Extended half-lifePromote recoveryPeptide/protein ingredientsAntibody mimetics/scaffoldsActivation peptideZymogen
The present invention relates to chimeric derivatives of serine protease zymogen containing the activation peptide of factor X or a fragment thereof for improving the half-life of said derivatives. Preferably, said chimeric derivatives are protein C and factor X derivatives. The invention also relates to said derivatives for the prevention or treatment of blood coagulation disorders.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM)
Compositions and methods to control bleeding
InactiveUS7015193B2Reduce needHigh activityFactor VIIPeptide/protein ingredientsMammalBlood coagulations
Owner:UNIVERSITY OF VERMONT
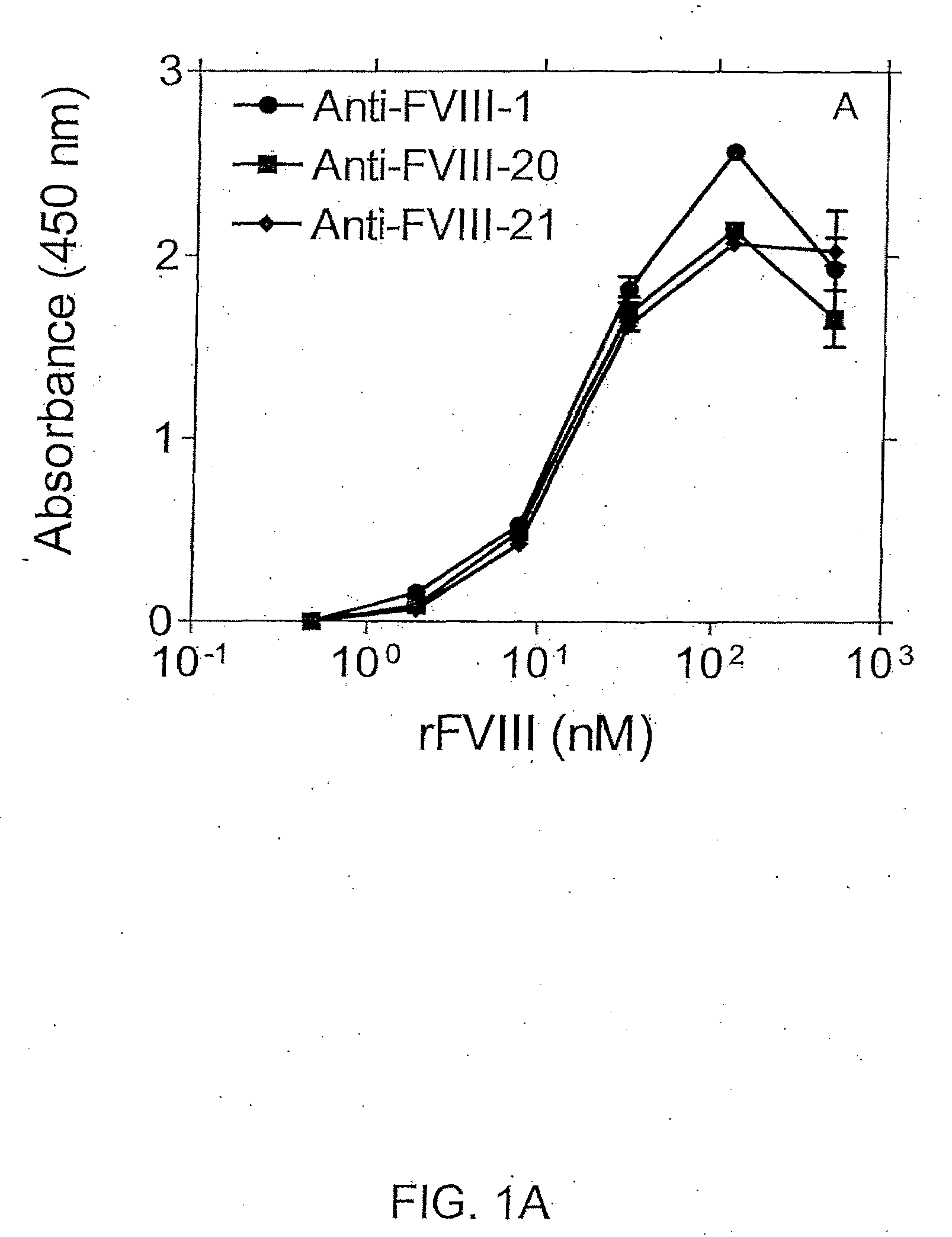
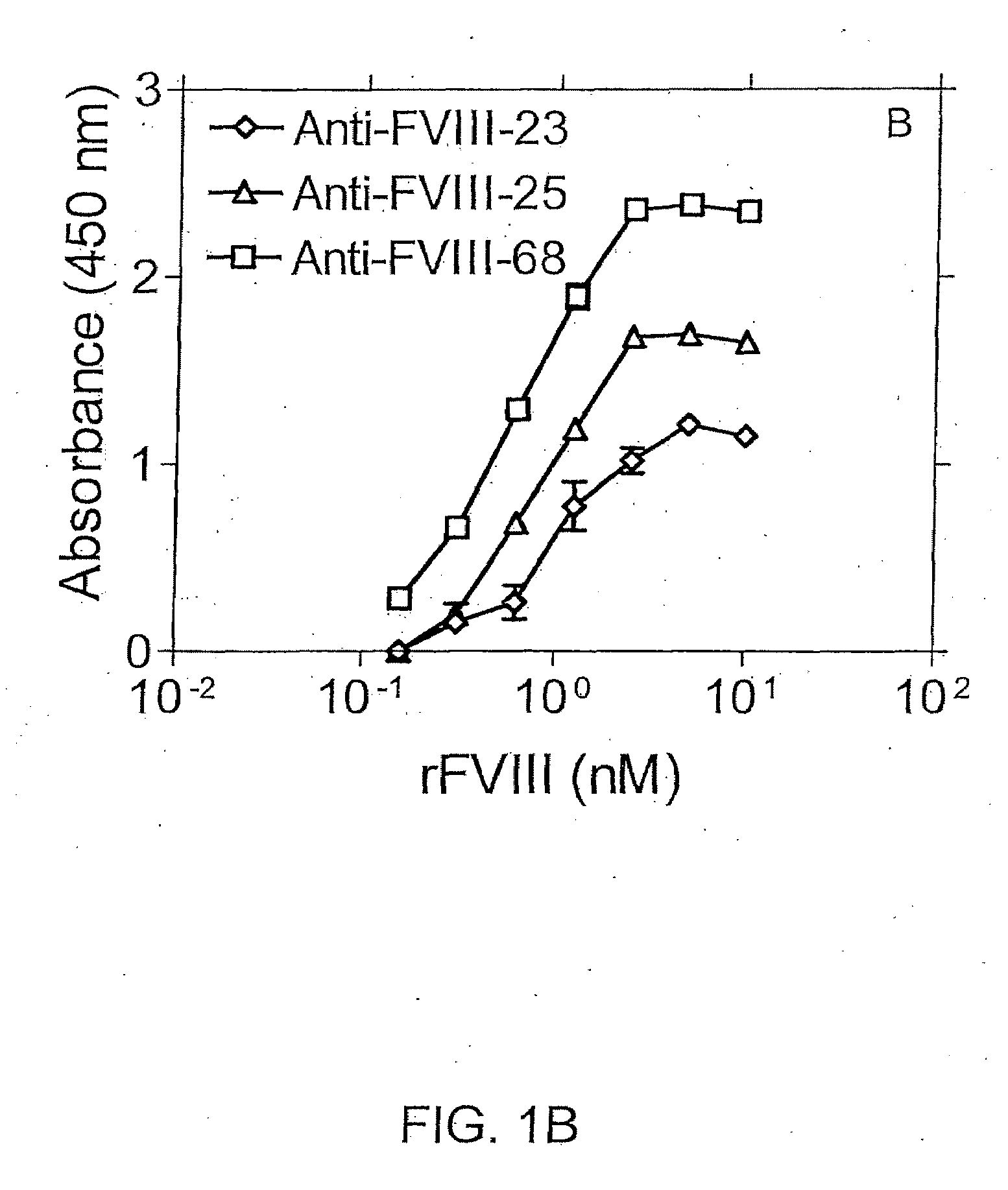
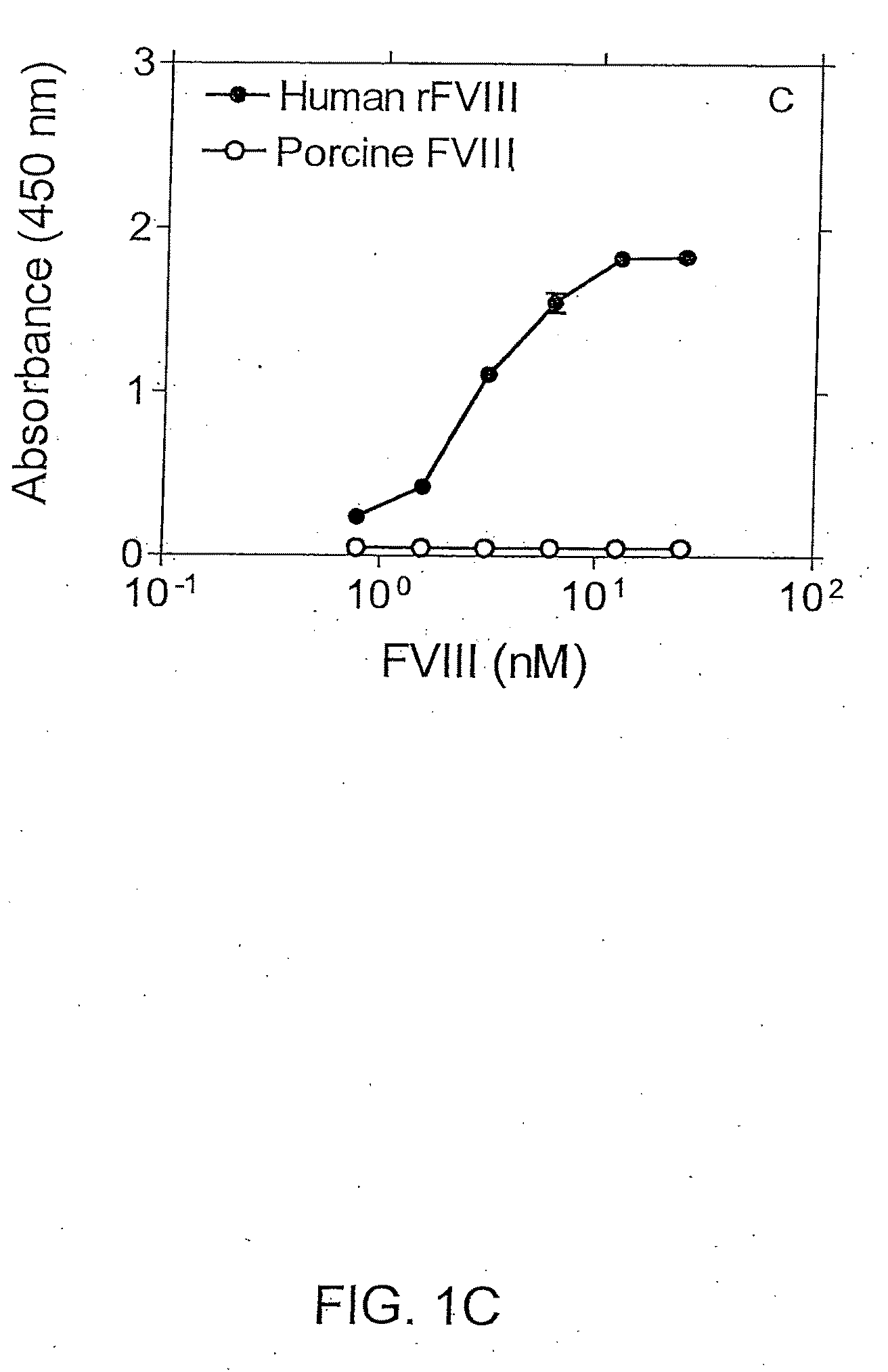
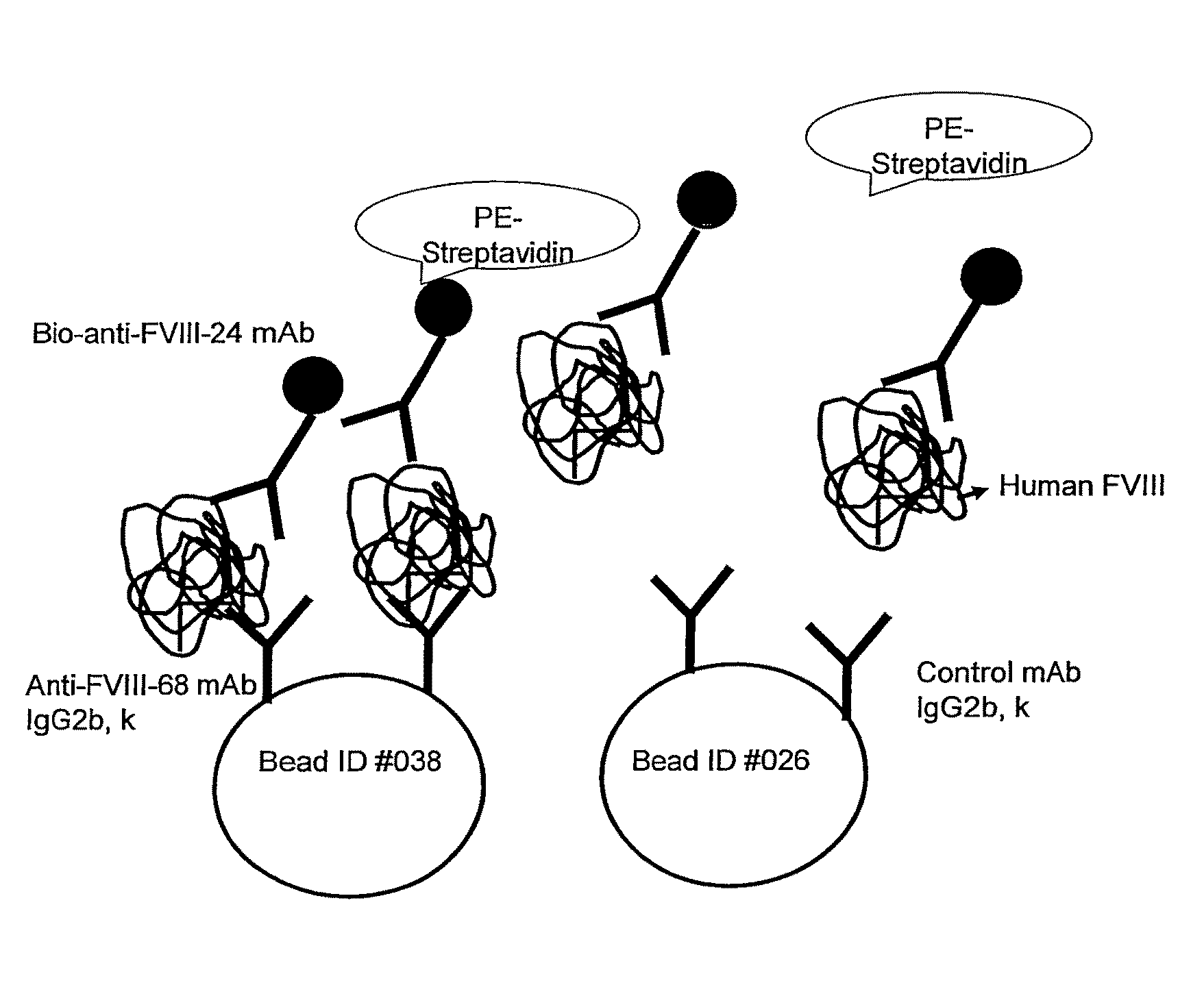
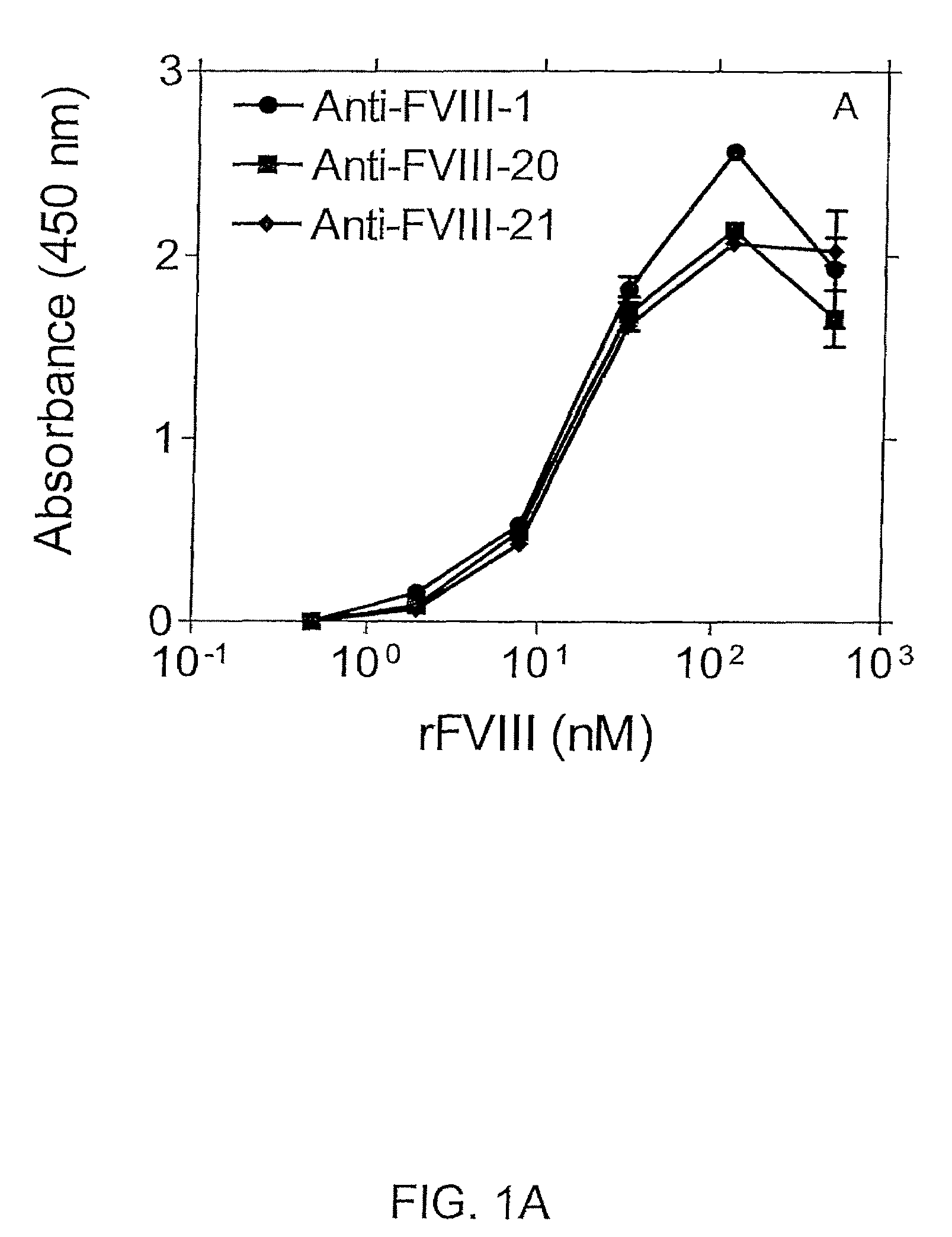
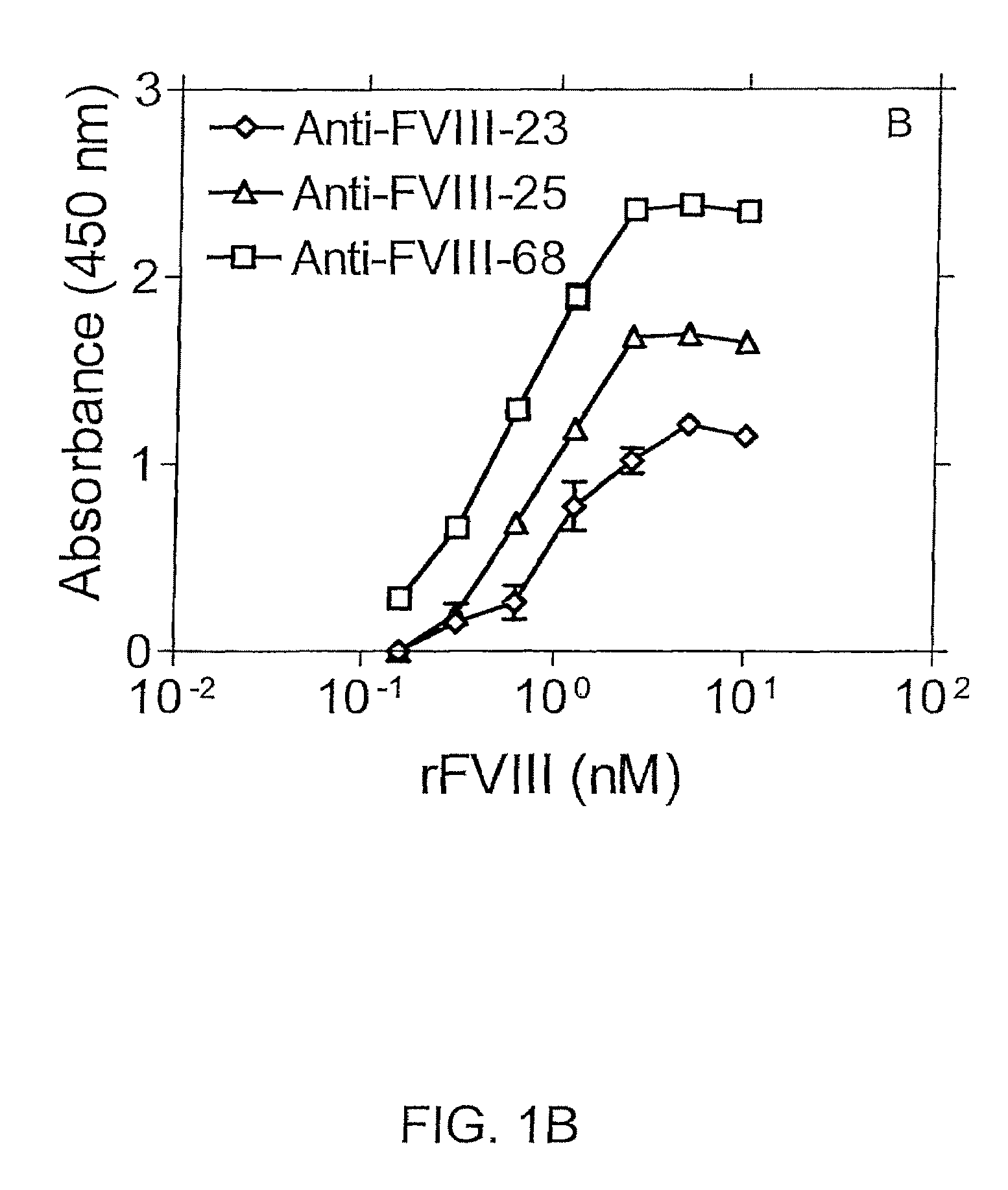
Highly Sensitive Immunoassays and Antibodies for Detection of Blood Factor VIII
InactiveUS20090215070A1Accurate detectionHigh sensitivityImmunoglobulins against blood coagulation factorsMicrobiological testing/measurementFactor iiHuman patient
Disclosed are antibodies that selectively bind to blood coagulation factor FVIII, and highly sensitive immunological assays comprising these antibodies. Preferred assays can detect FVIII at about 3500-fold below the normal physiological levels, and have a wide array of applications including accurate monitoring of FVIII concentration in pharmaceutical products for treatment of blood coagulation disorders, and determination of FVIII levels in plasma of human patients, including those with blood coagulation disorders such as hemophilia.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
Highly sensitive immunoassays and antibodies for detection of blood factor VIII
InactiveUS8236518B2Immunoglobulins against blood coagulation factorsMicrobiological testing/measurementFactor iiHuman patient
Disclosed are antibodies that selectively bind to blood coagulation factor FVIII, and highly sensitive immunological assays comprising these antibodies. Preferred assays can detect FVIII at about 3500-fold below the normal physiological levels, and have a wide array of applications including accurate monitoring of FVIII concentration in pharmaceutical products for treatment of blood coagulation disorders, and determination of FVIII levels in plasma of human patients, including those with blood coagulation disorders such as hemophilia.
Owner:MAYO FOUND FOR MEDICAL EDUCATION & RES
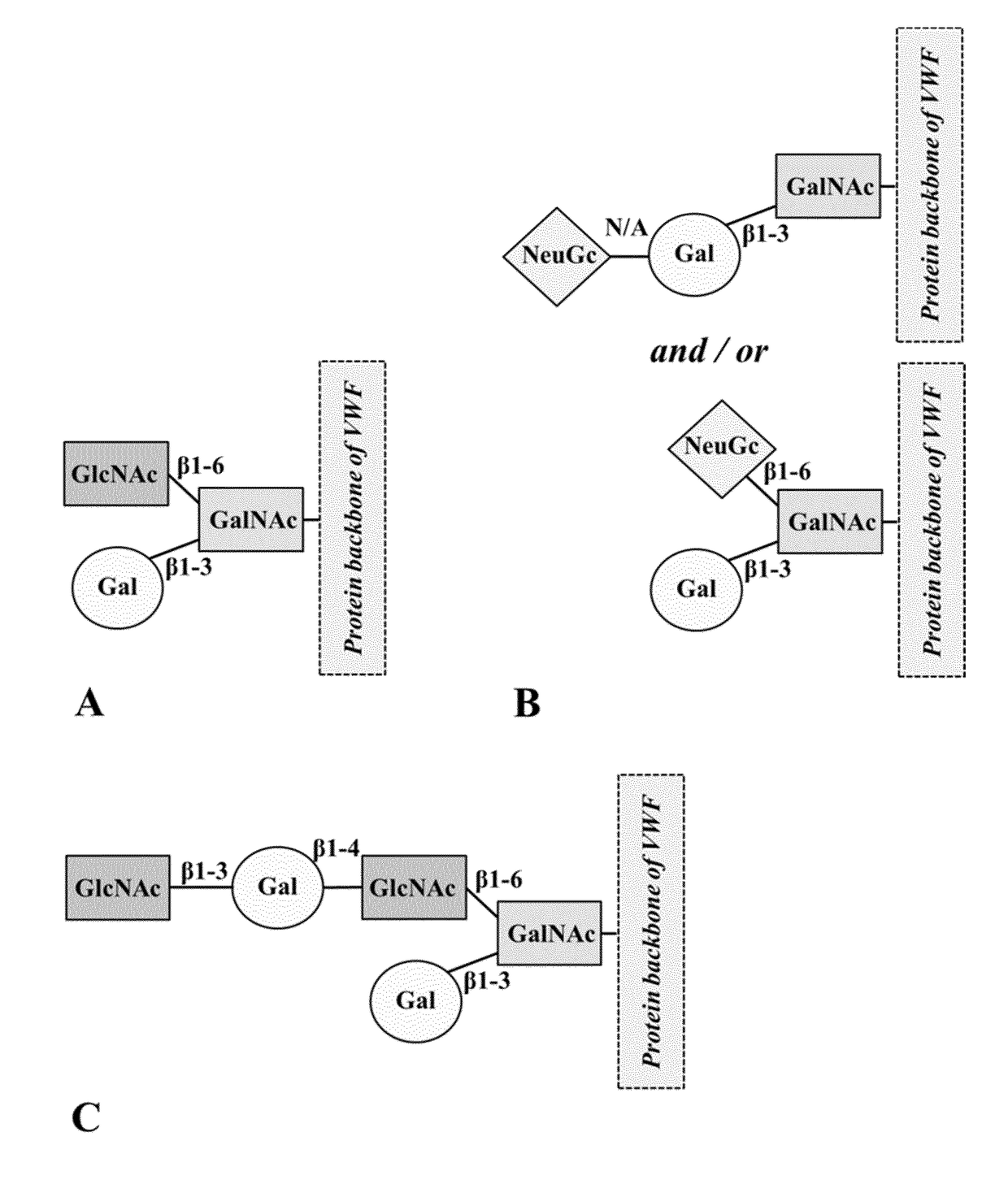
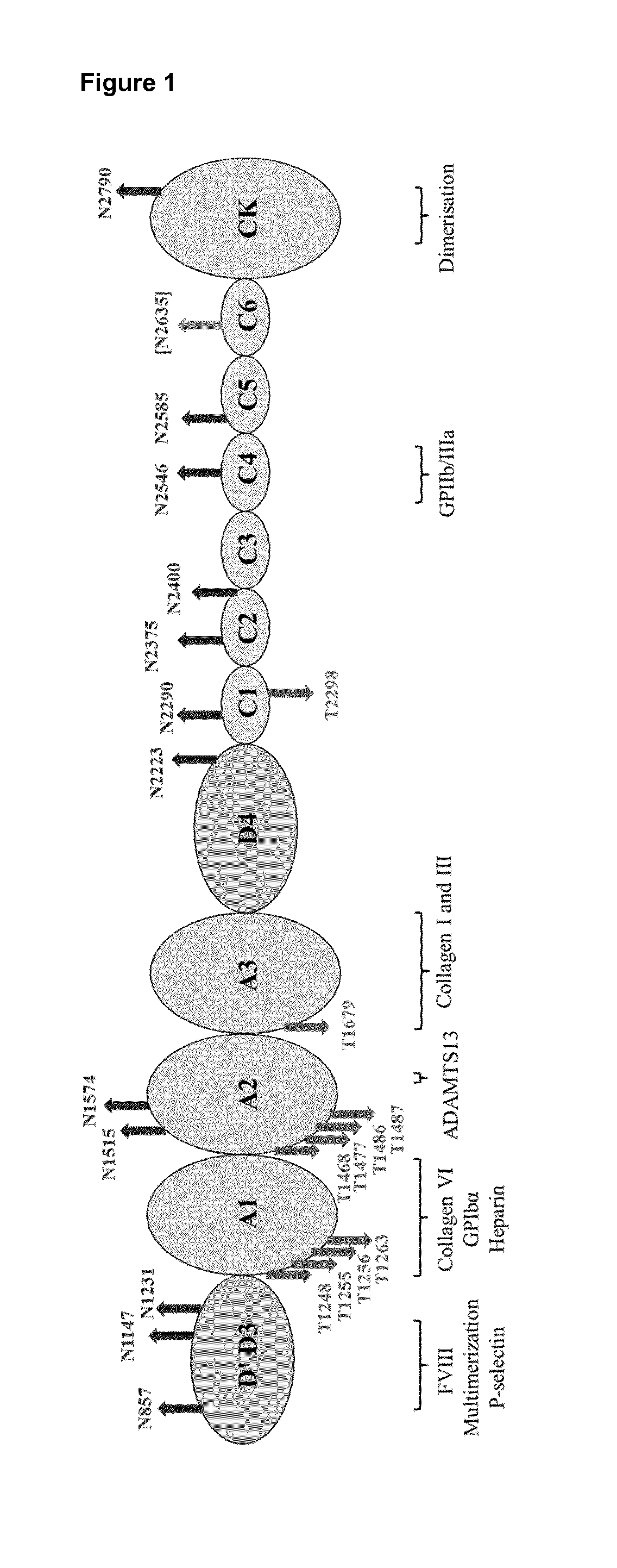
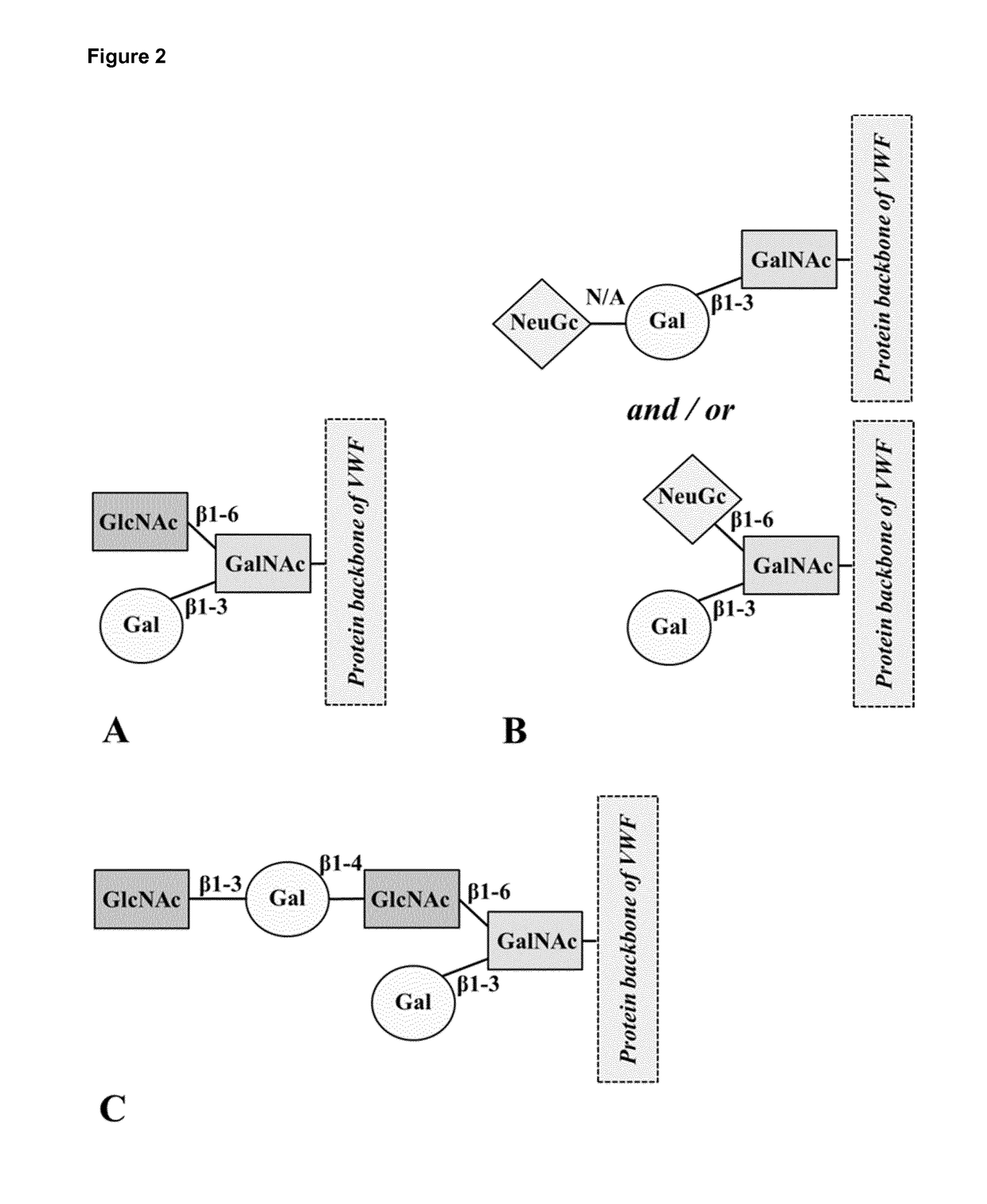
Truncated von willebrand factor polypeptides for treating hemophilia
PendingCN109922824AReduced activity levelIncrease activity levelFactor VIIPeptide/protein ingredientsFactor VIII vWFBlood plasma
The invention pertains to a polypeptide comprising a truncated von Willebrand Factor (VWF) and a half-life extending moiety, for use in the treatment of a blood coagulation disorder, said treatment comprising administering the polypeptide to a subject having a blood coagulation disorder and having endogenous Factor VIII (FVIII), wherein the activity level of endogenous FVIII in said subject beforetreatment with said polypeptide is reduced relative to the activity level of FVIII in normal human plasma (NHP) provided that the activity level of endogenous FVIII in said subject is at least 0.5% of the activity level of endogenous FVIII in normal human plasma (NHP), wherein the polypeptide is capable of binding to endogenous FVIII and wherein the endogenous FVIII level is increased following administration of said polypeptide.
Owner:CSL BEHRING LENGNAU AG
Truncated von willebrand factor polypeptides for treating hemophilia
ActiveUS20180161402A1Extended half-lifeReduce dosing frequencyFactor VIIPeptide/protein ingredientsFactor VIII vWFHalf-life
The invention relates to a polypeptide comprising a truncated von Willebrand Factor (VWF) for use in the treatment of a blood coagulation disorder, wherein the polypeptide carries a half-life extending moiety and is administered in molar excess over Factor VIII and / or endogenous VWF.
Owner:CSL BEHRING LENGNAU AG
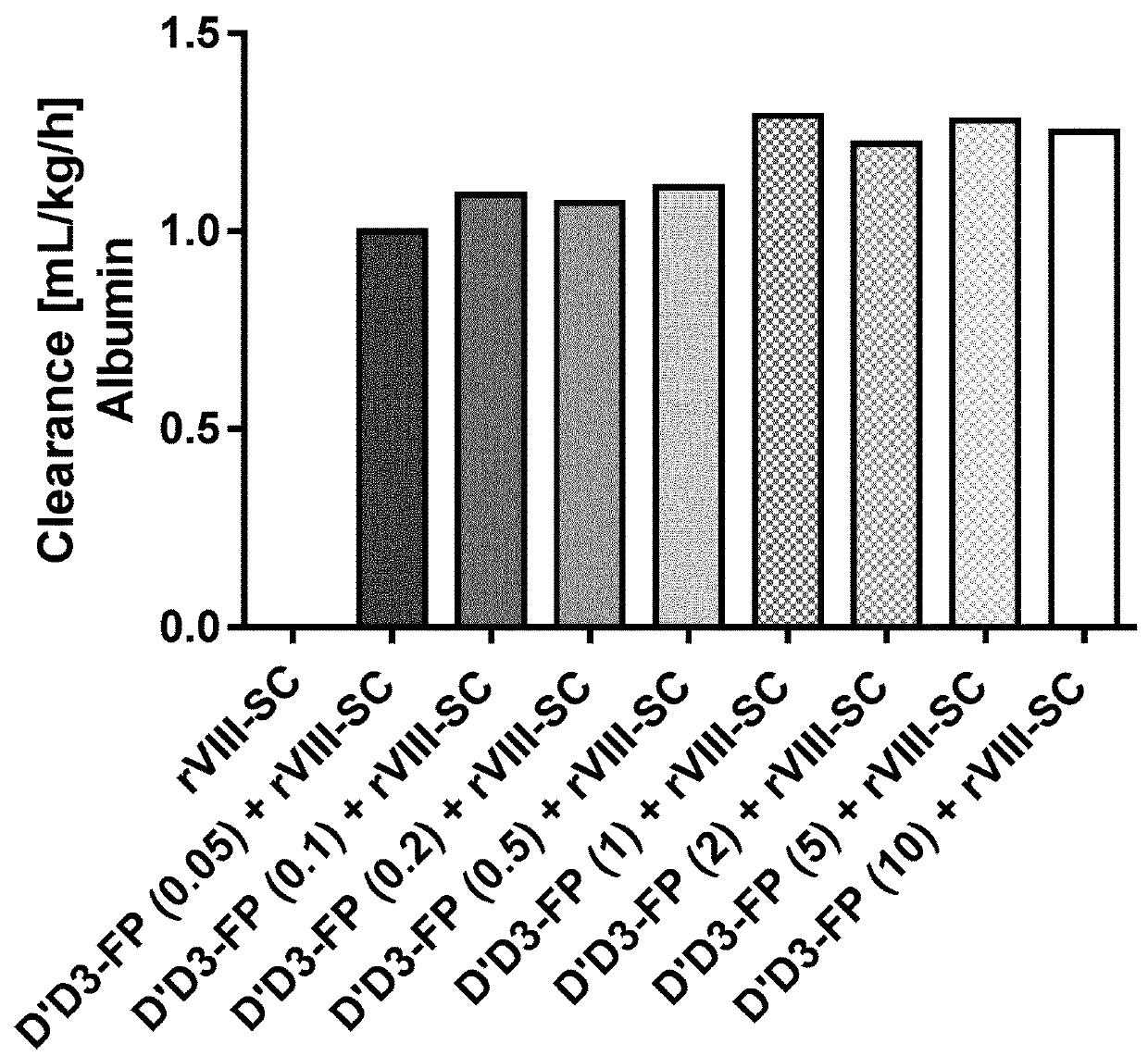
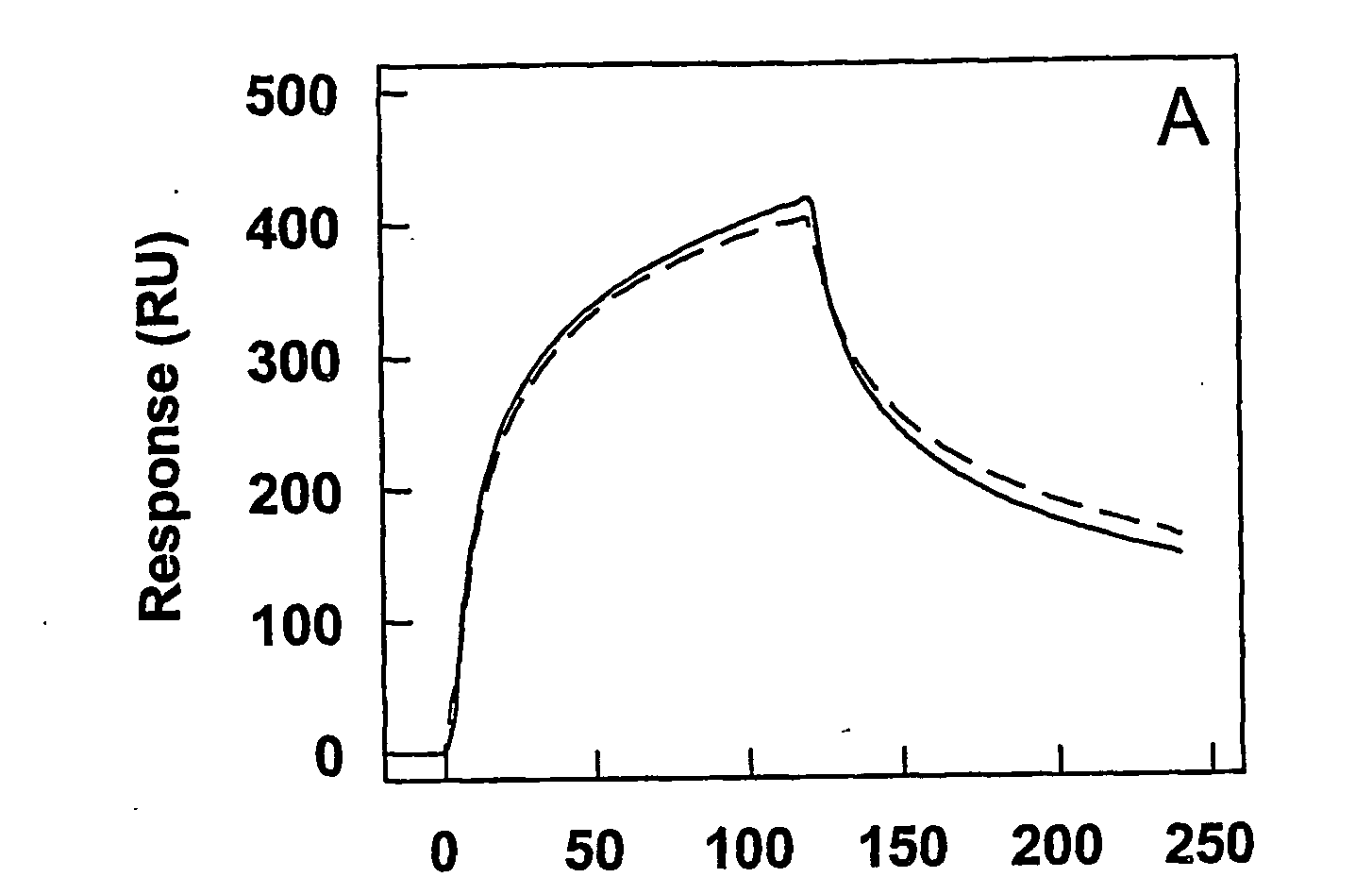
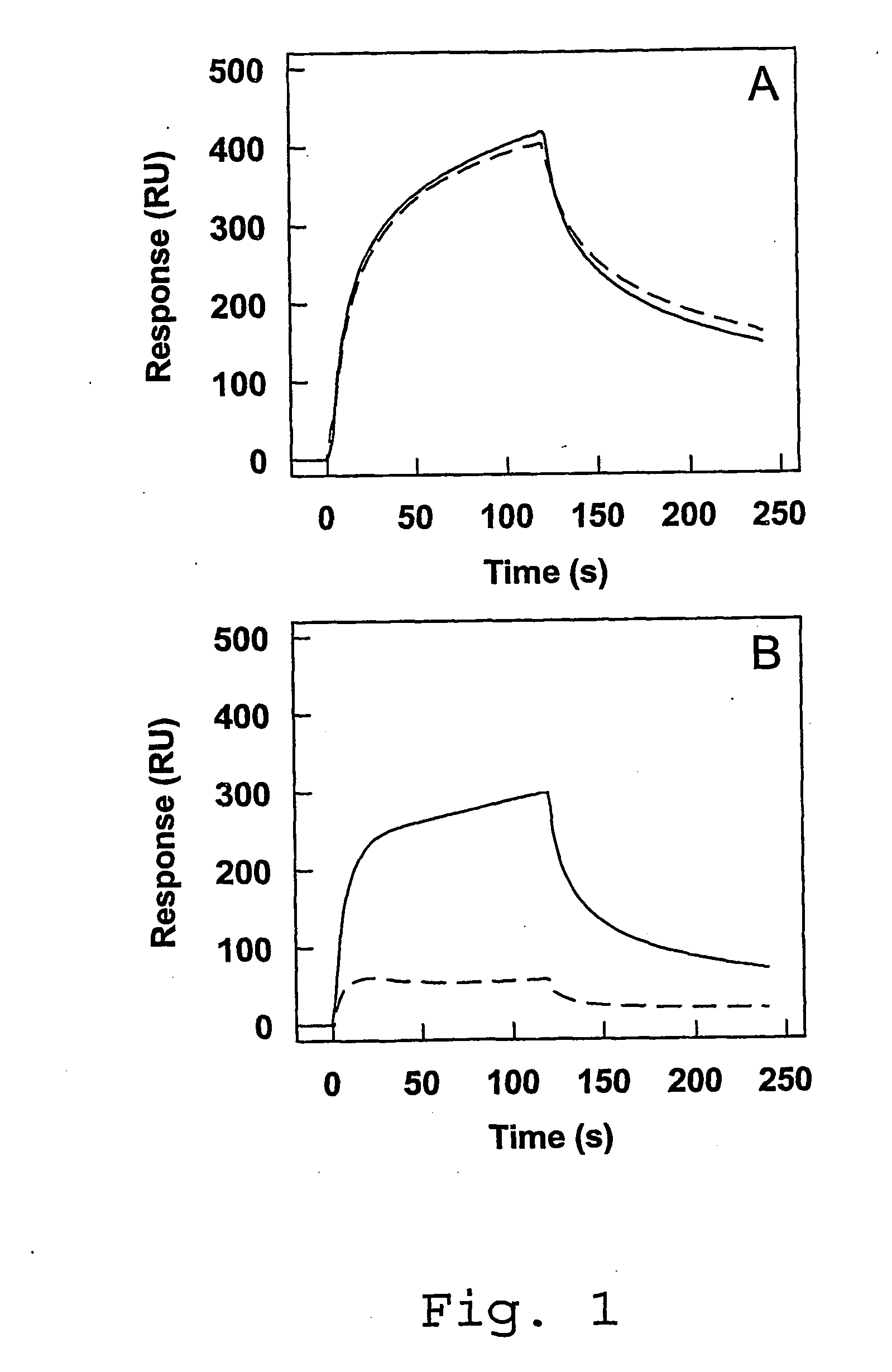
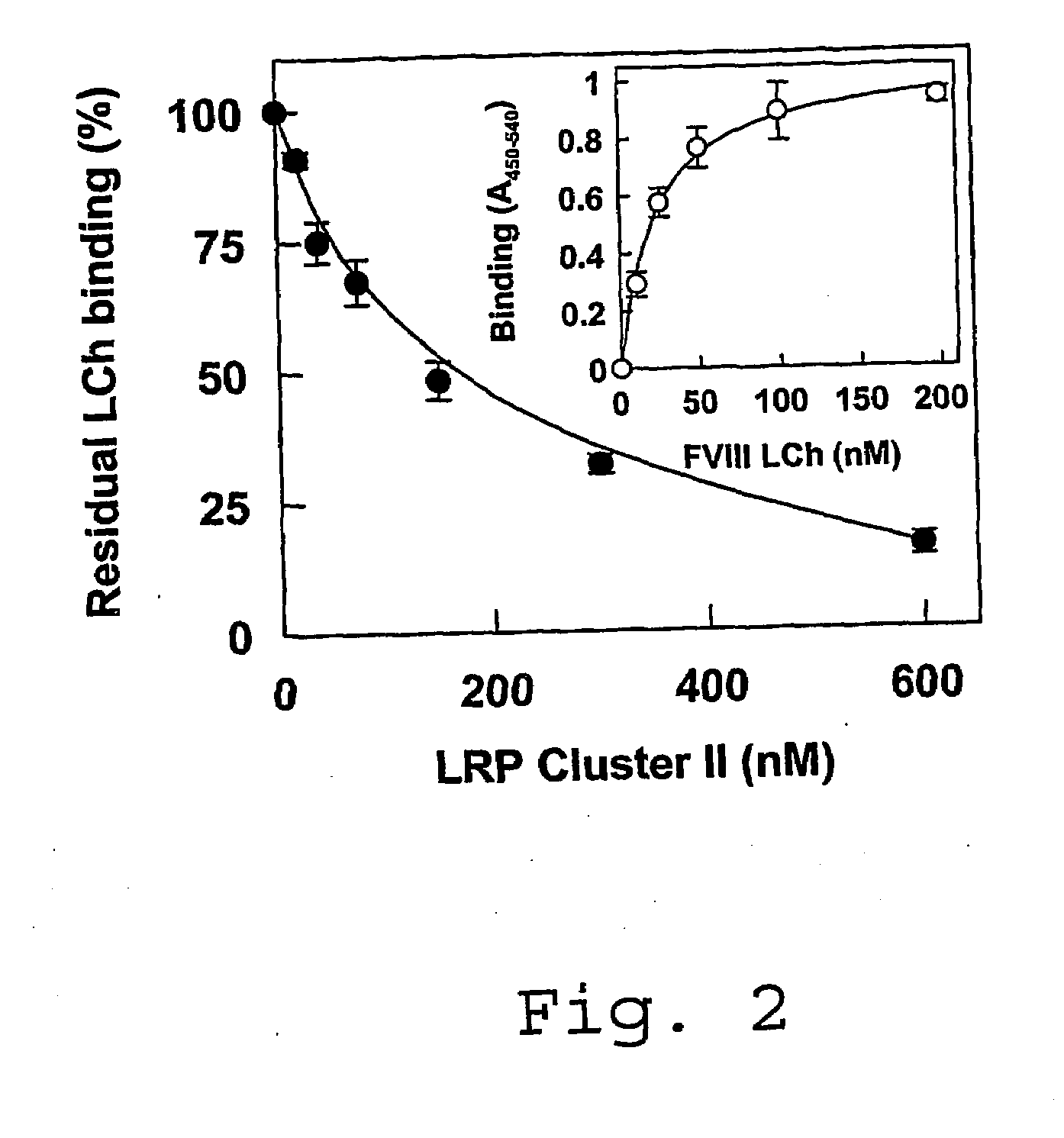
Antagonists of Factor VIII Interaction with Low-Density Lipoprotein Receptor Related Protein
ActiveUS20080219983A1Improve stabilityYields using current plasma fractionation methods are lowImmunoglobulins against blood coagulation factorsFactor VIIHalf-lifeFactor ii
The present invention concerns the use of peptides derived from and antibodies generated against Factor VIII and the inhibition of Factor VIII interaction with LRP. Furthermore, the present invention concerns a method to inhibit LRP interaction with Factor VIII as well as a method to decrease Factor VIII degradation and / or prolong Factor VIII half-life in a biological fluid and / or a method to treat patients suffering from a blood coagulation disorder, especially Haemophilia A. The present invention also concerns a pharmaceutical composition useful for the decrease of Factor VIII degradation in a biological fluid, the inhibition of Factor VIII interaction with LRP, and / or the prolongation of Factor VIII half-life in a biological fluid for treatment of a blood coagulation disorder, especially Haemophilia A.
Owner:STICHTING SANQUIN BLOEDVOORZIENING
Modified von willebrand factor having improved half-life
ActiveUS20180051067A1Extended half-lifeFactor VIIPeptide/protein ingredientsFactor VIII vWFHalf-life
The invention relates to a modified VWF molecule for use in the treatment of a blood coagulation disorder.
Owner:CSL BEHRING LENGNAU AG
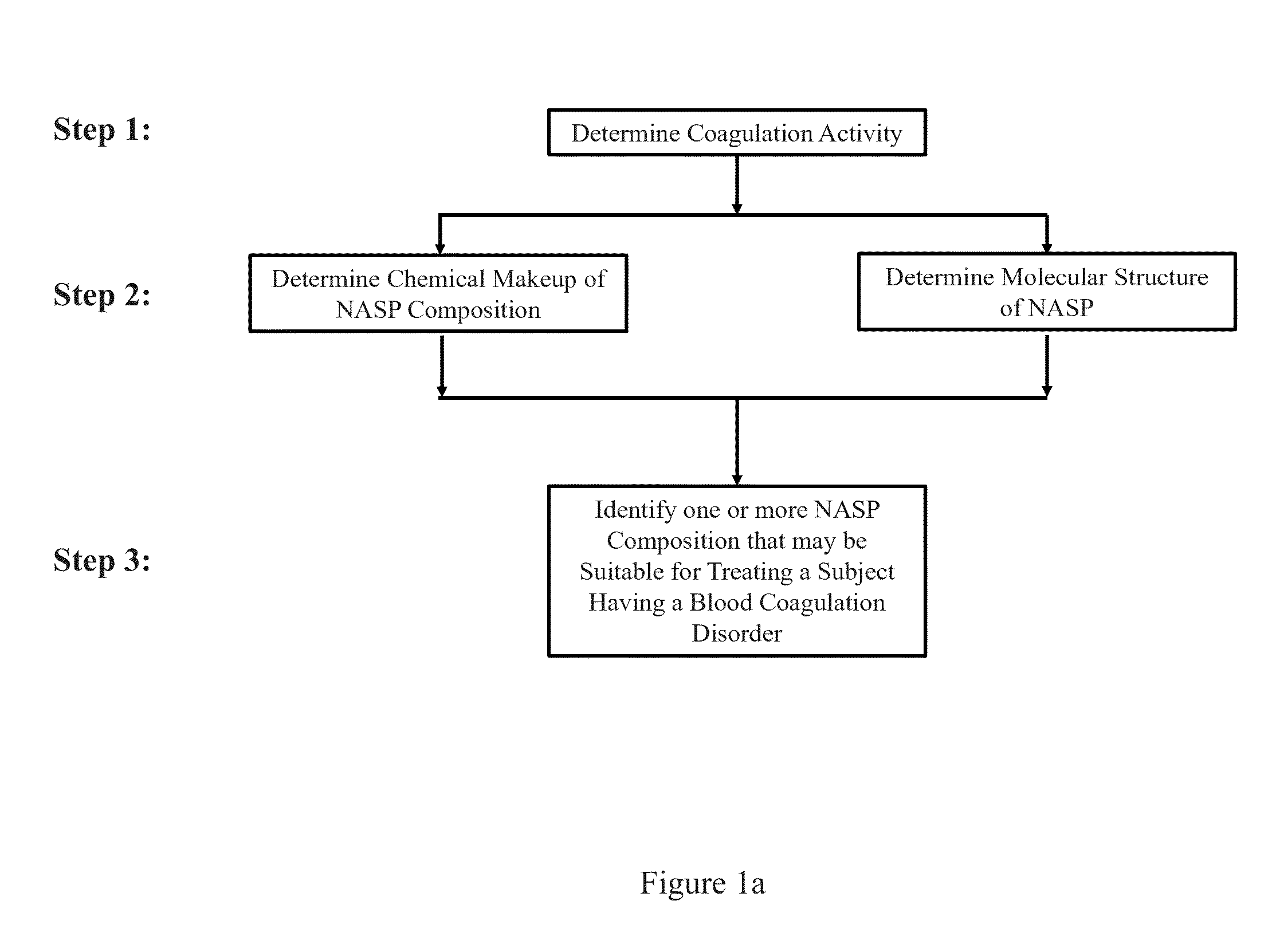
Methods and systems for screening compositions comprising non-anticoagulant sulfated polysaccharides
ActiveUS20140051657A1Prolong clotting timeOrganic active ingredientsBiocideSulfated polysaccharidesMedicine
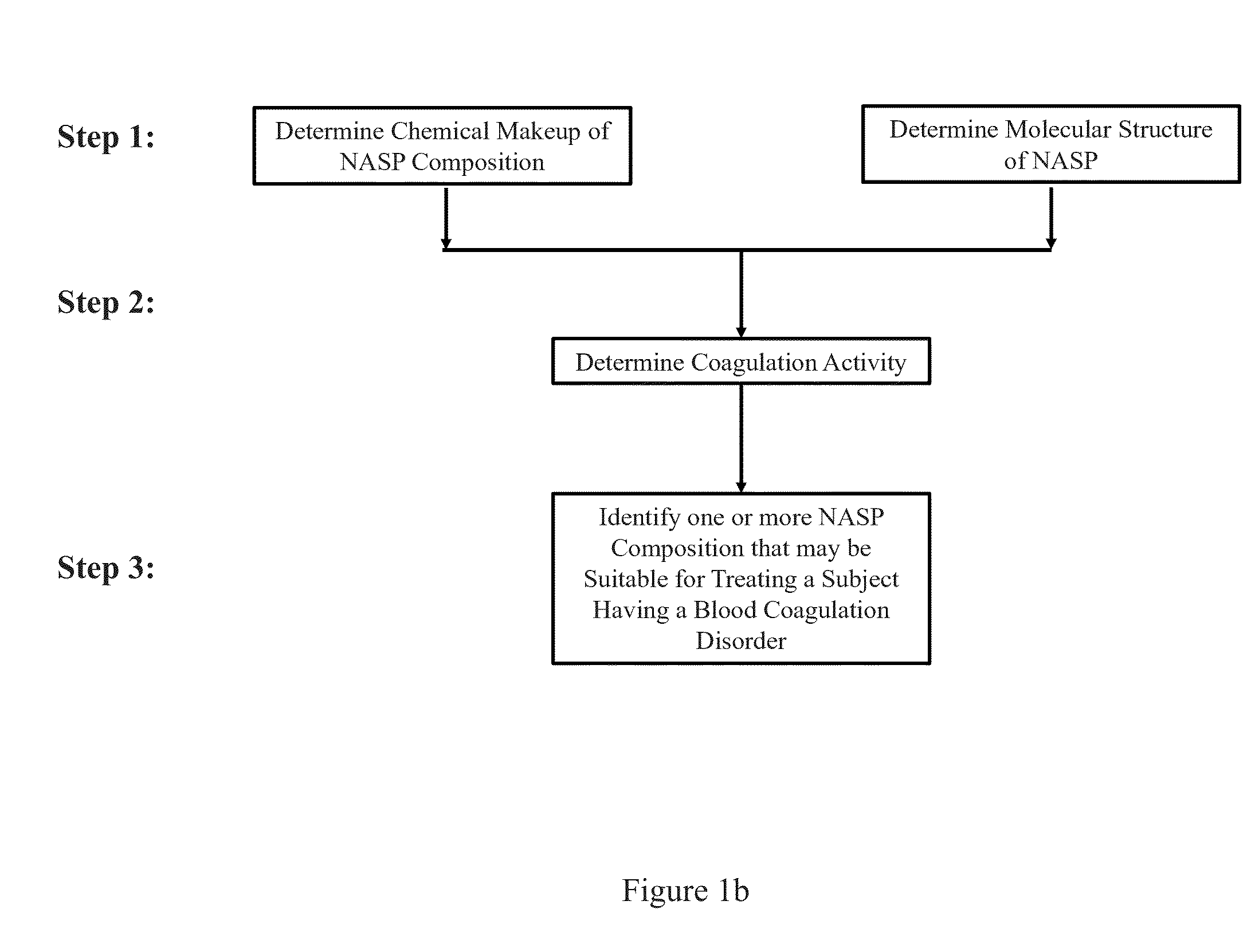
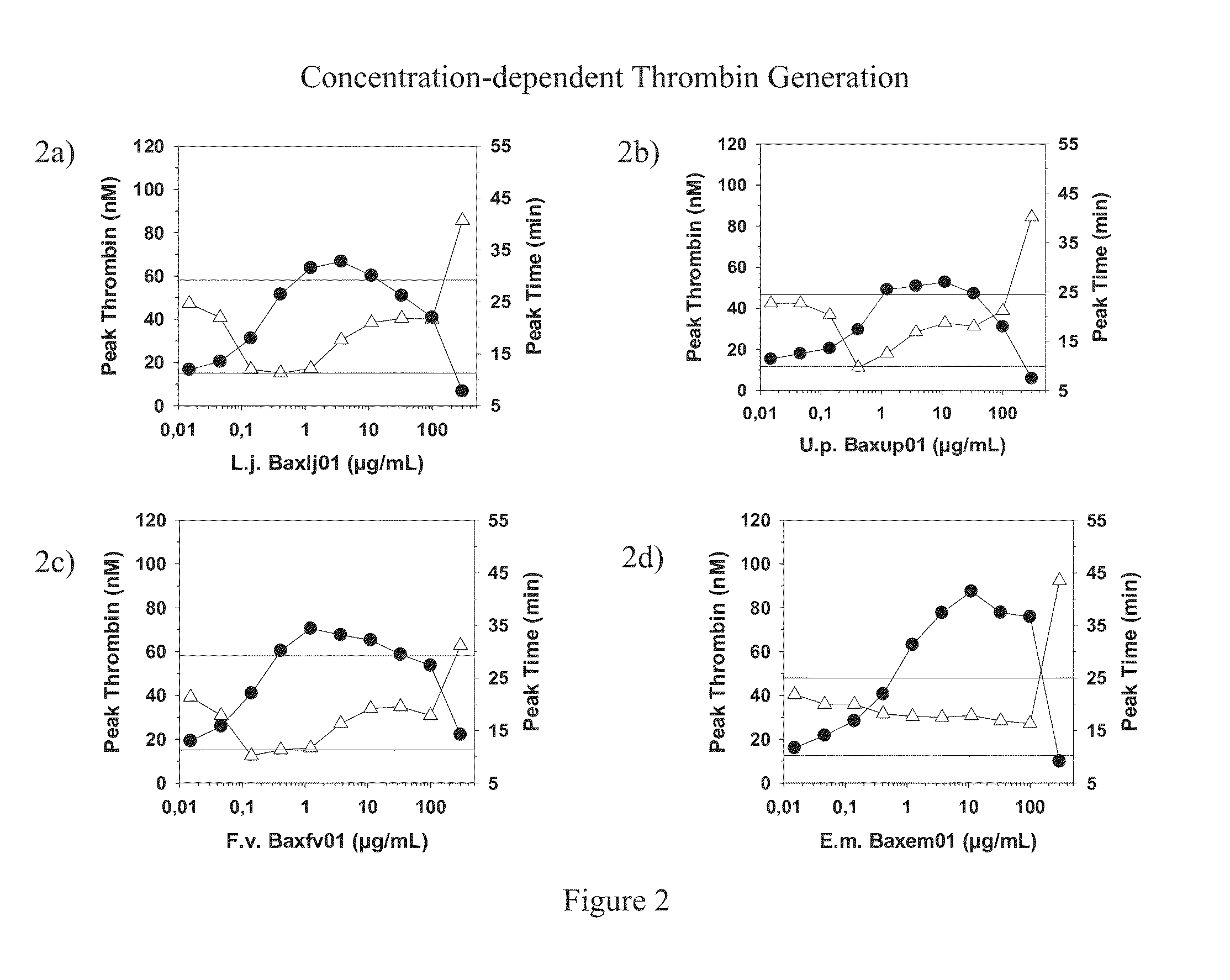
Aspects of the invention include methods for identifying one or more NASP (non-anticoagulant sulfated polysaccharide) compositions that are suitable for treating a subject having a blood coagulation disorder. In practicing methods according to certain embodiments, NASP compositions are evaluated by determining the coagulation activity and chemical makeup of the NASP composition and the molecular structure of the NASP. Systems for practicing methods of the invention as well as compositions suitable for treating a subject having a blood coagulation disorder are also described.
Owner:BAXALTA GMBH
Methods and devices for detection of anticoagulants in plasma and whole blood
PendingCN111094990AUnderstanding PathophysiologyPeptide/protein ingredientsLaboratory glasswaresAnticoagulant AgentAnti coagulation
Methods and devices for evaluating coagulation are described, including methods and devices for detecting an anticoagulant agent or a coagulation abnormality. In various embodiments, the methods and devices of the invention measure the coagulation of a sample in response to a gradient of one or more coagulation factors. These responses can be evaluated to accurately profile the coagulation impairments of the sample, including the presence of anticoagulant medication. In various embodiments, the invention provides the point-of-care or bedside testing with a convenient, microfluidic device thatcan be used by the minimally trained personnel.
Owner:MASSACHUSETTS INST OF TECH +1
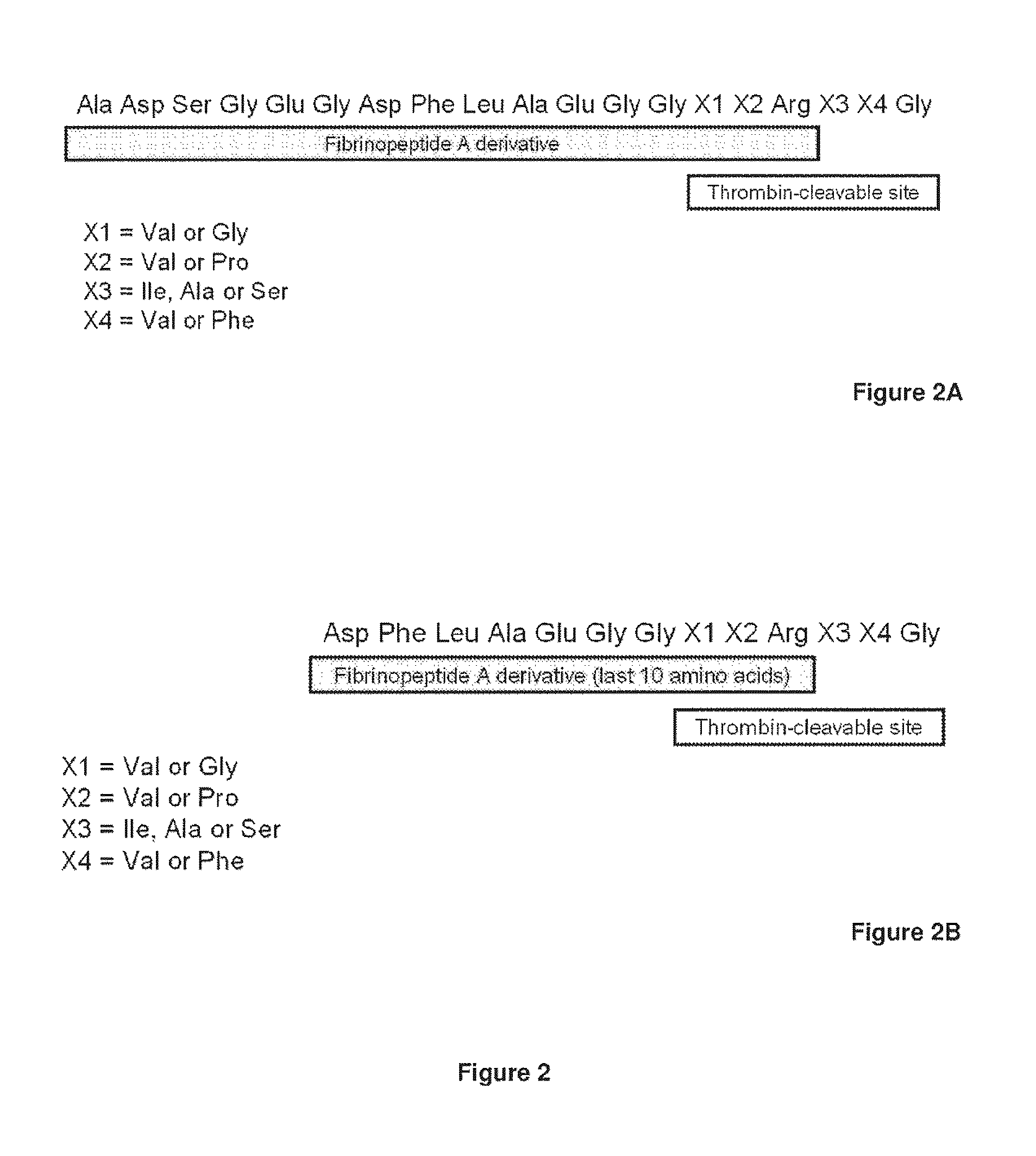
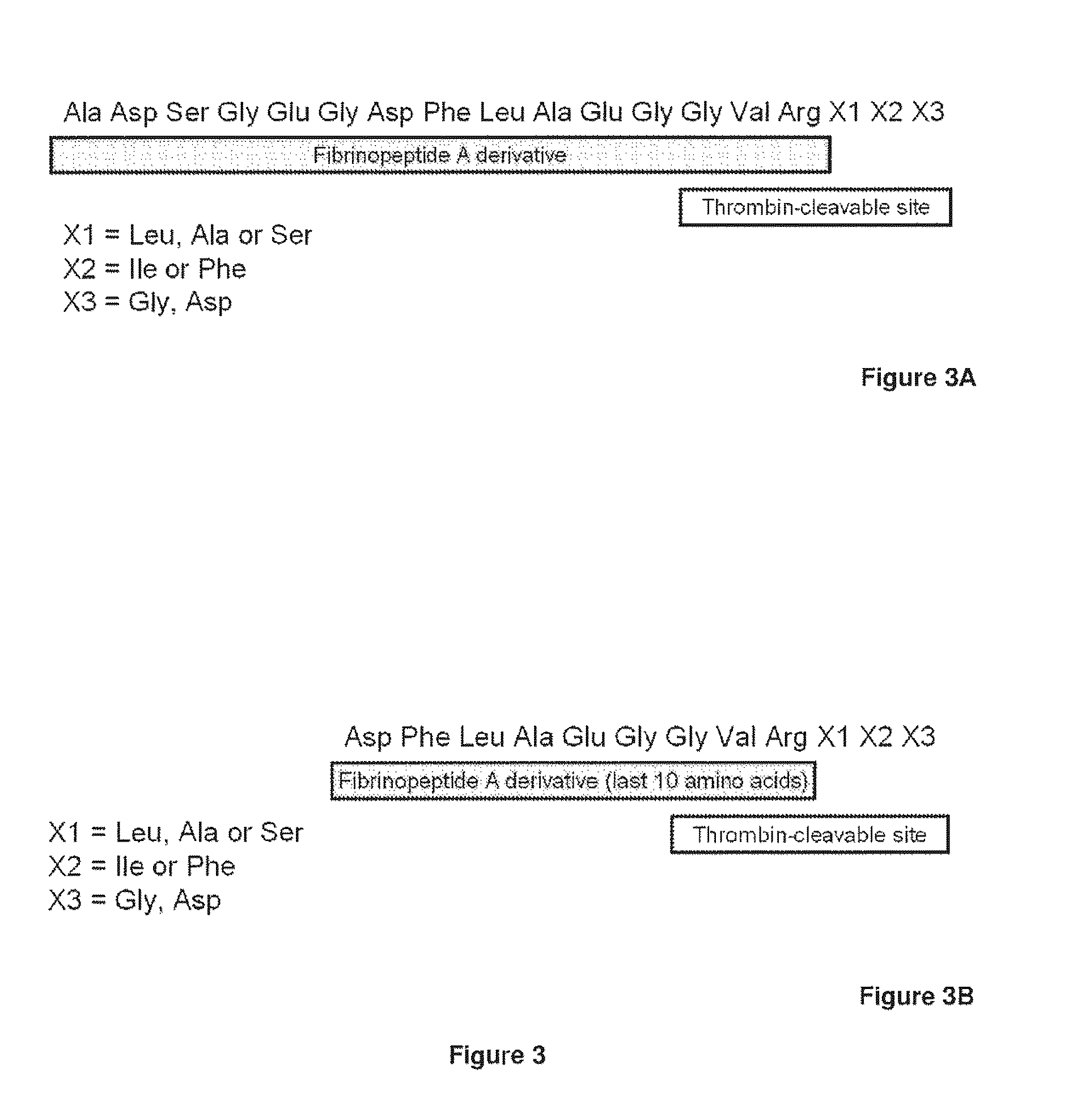
Exosite-Directed Thrombin Inhibitors
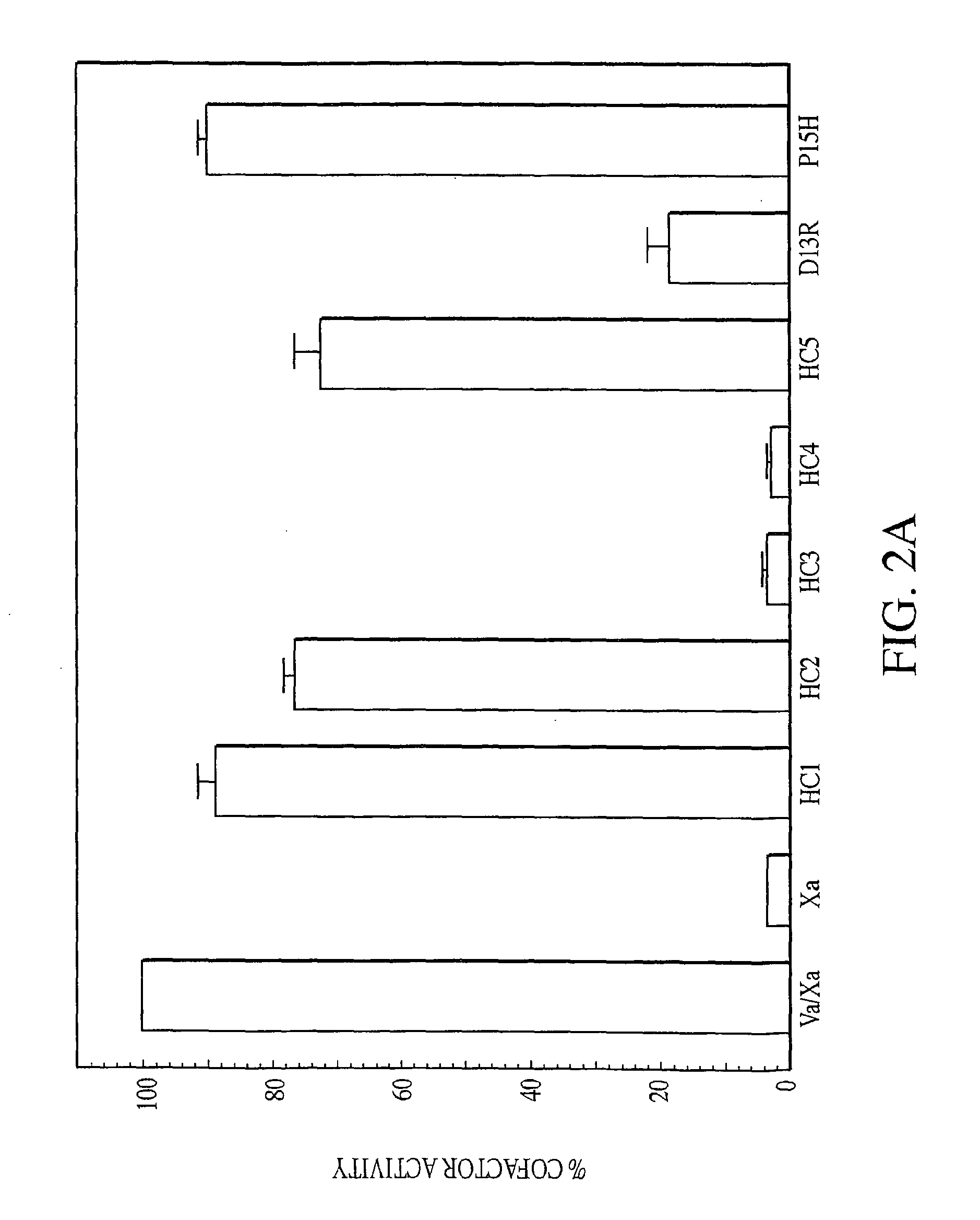
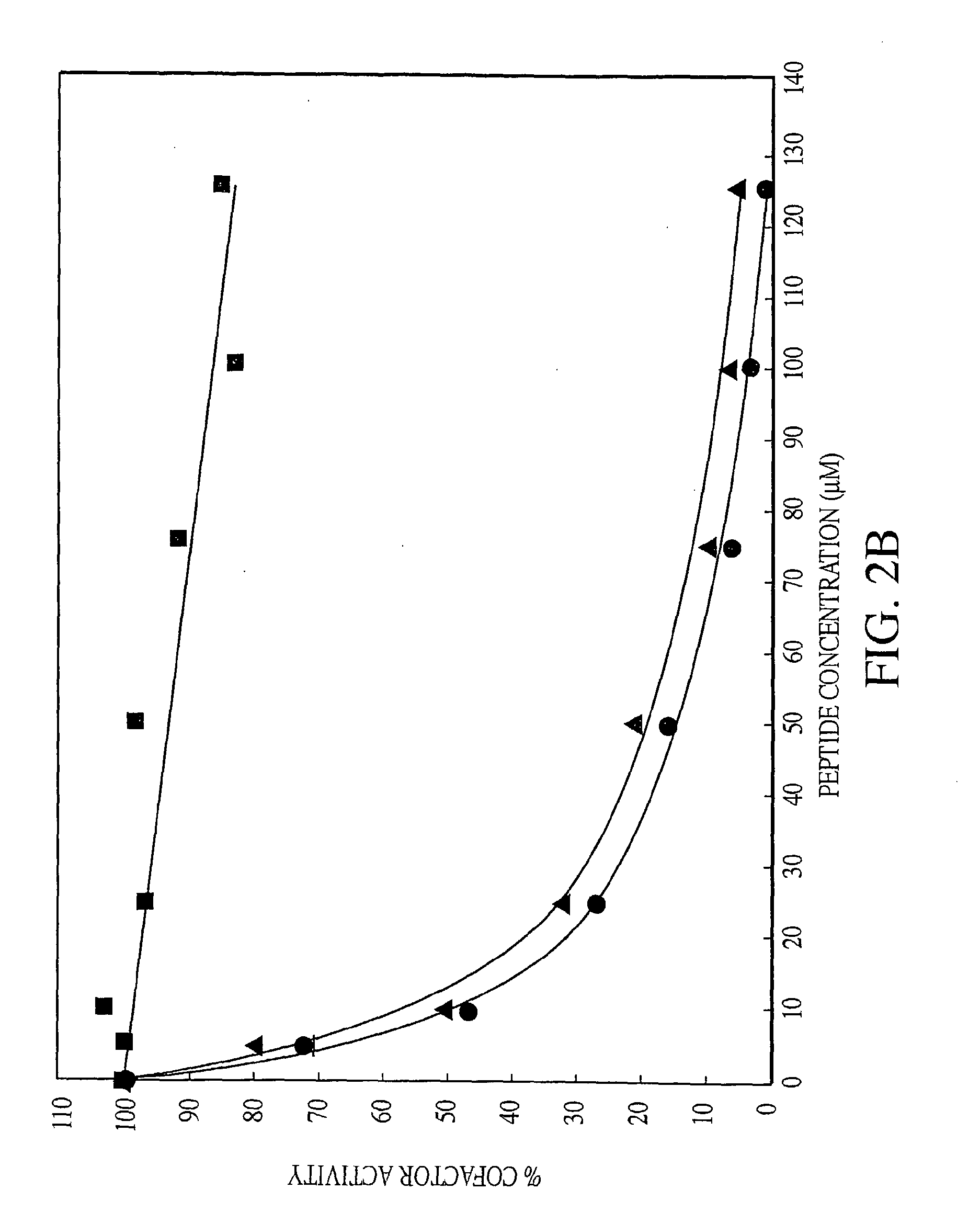
Disclosed are an amino acid sequence of the human blood clotting factor Va, peptides containing such sequence, and additional peptides of interest that significantly inhibit thrombin generation. Also disclosed are pharmaceutical compositions containing these peptides and related therapeutic methods for inhibiting thrombin generation and treating blood coagulation disorders.
Owner:KALAFATIS MICHAEL
Kits for screening for blood coagulation disorders
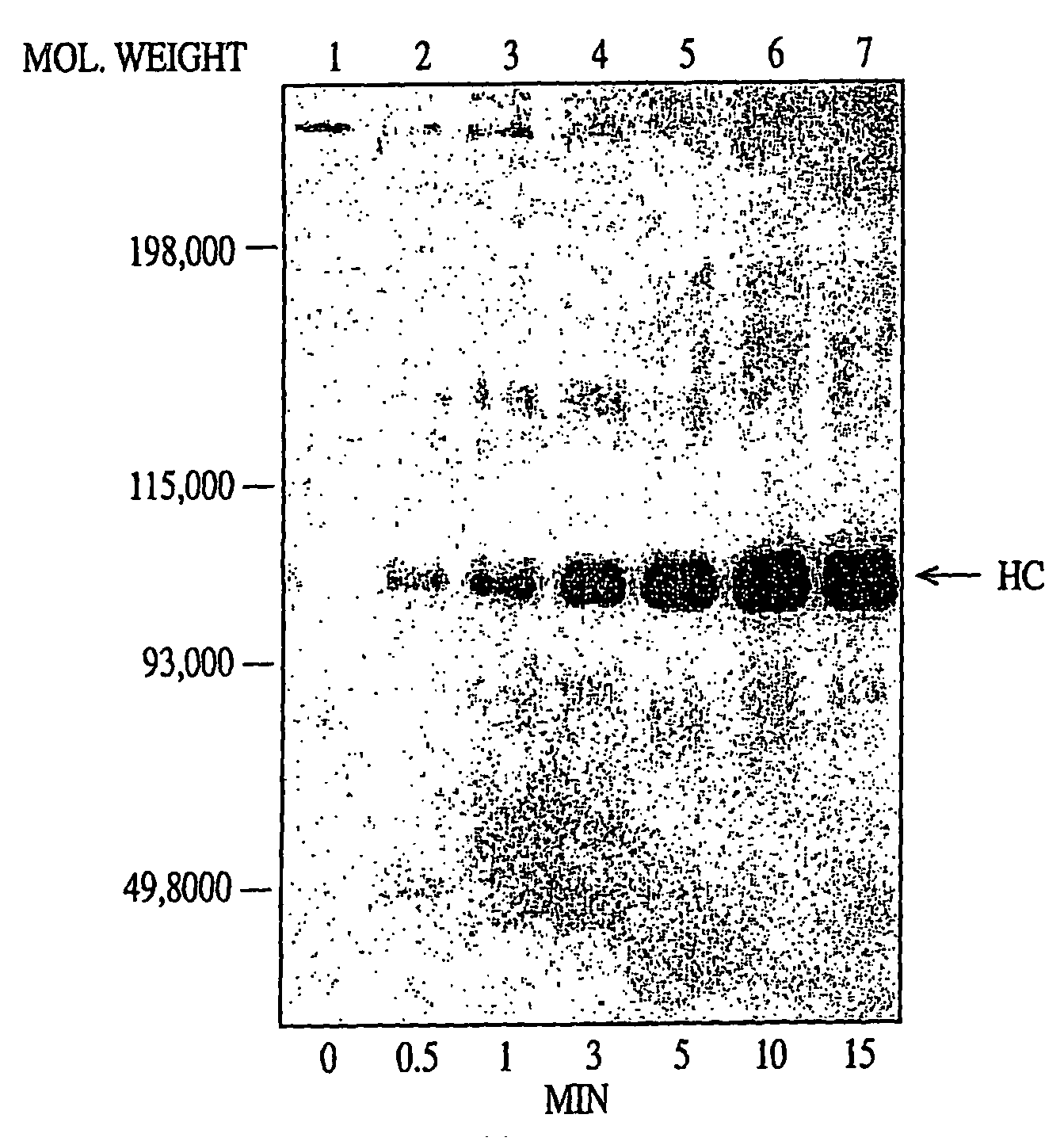
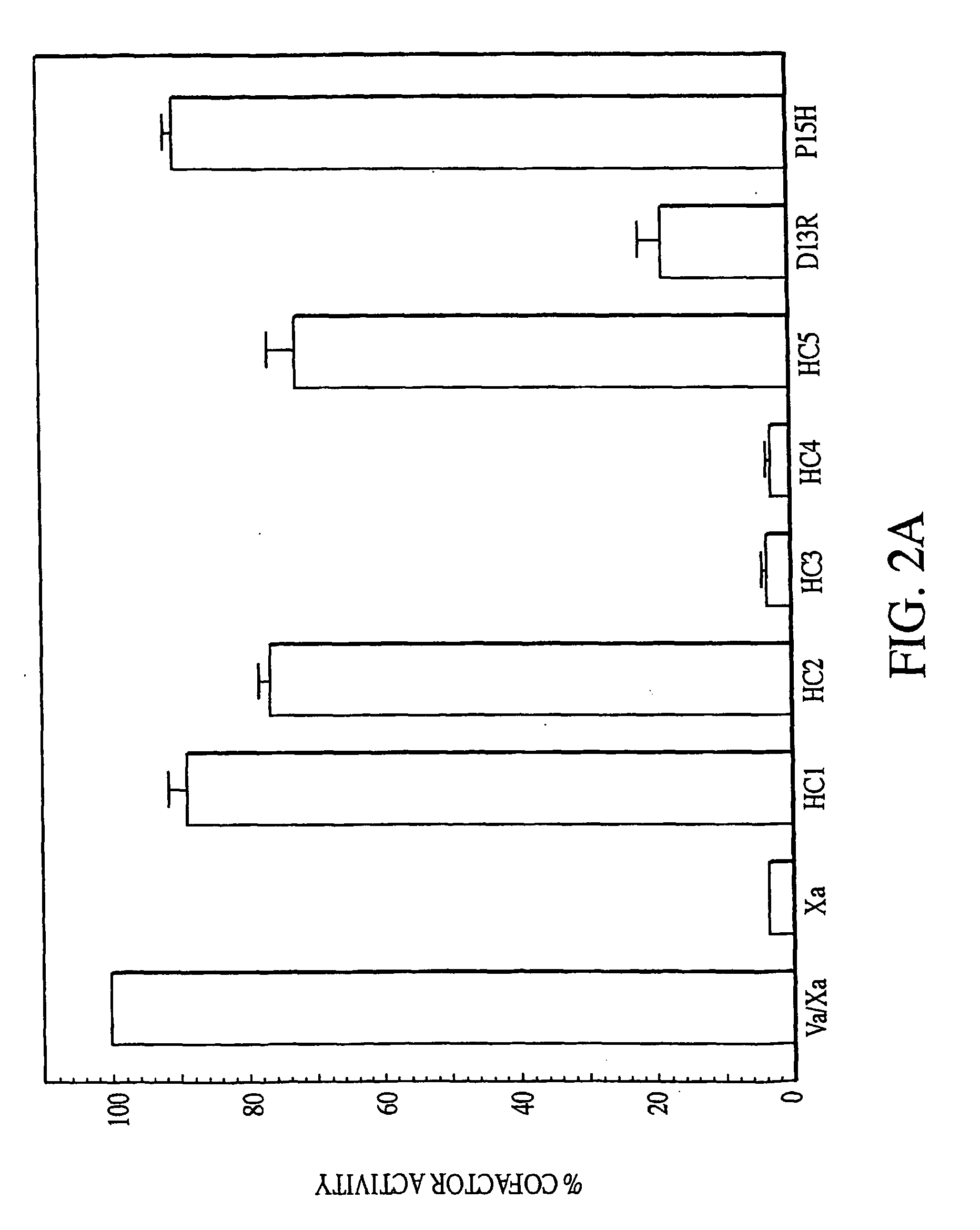
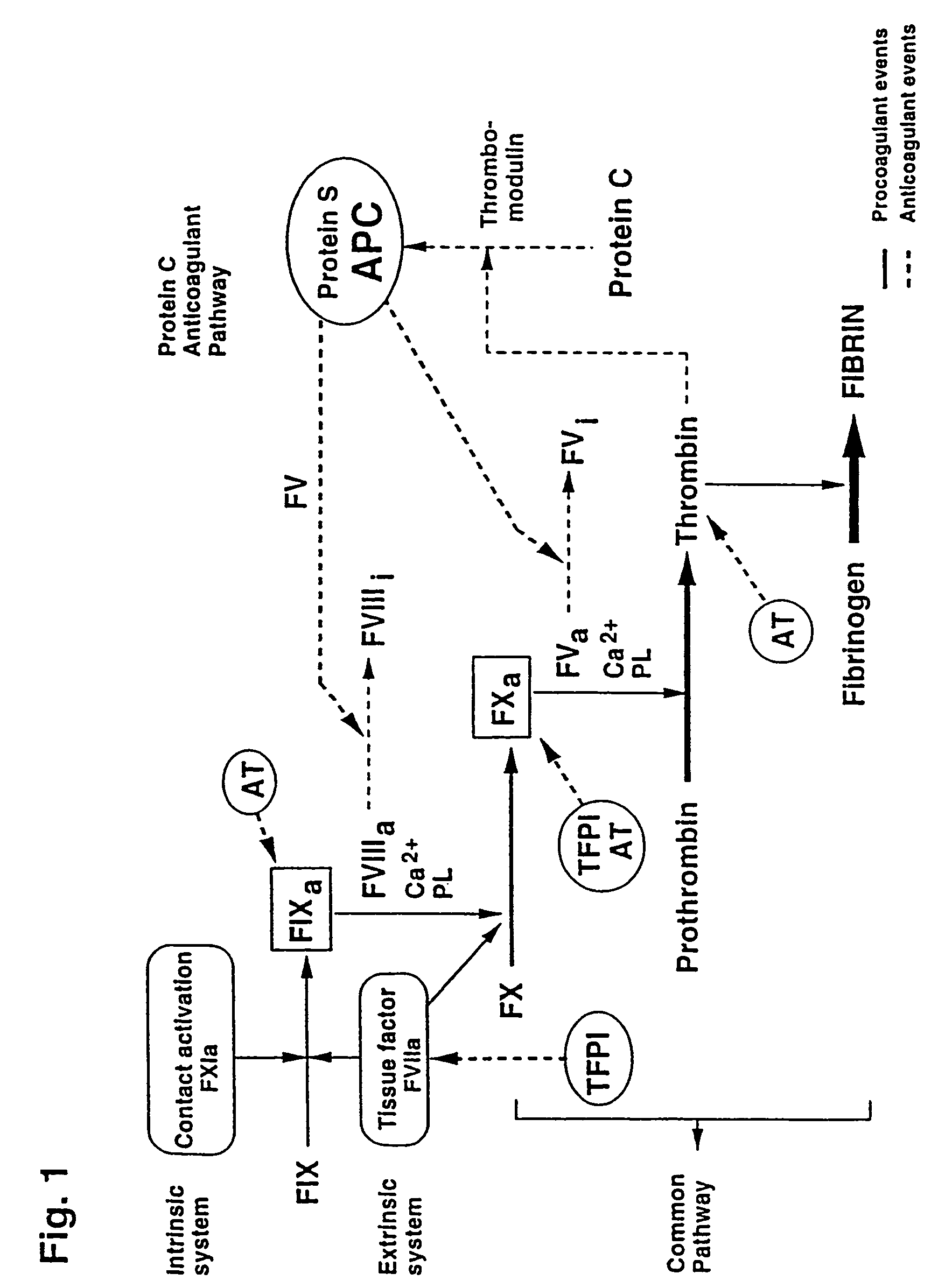
InactiveUS7118880B2Improve stabilityMicrobiological testing/measurementEnzymologyFunctional activityBiological activation
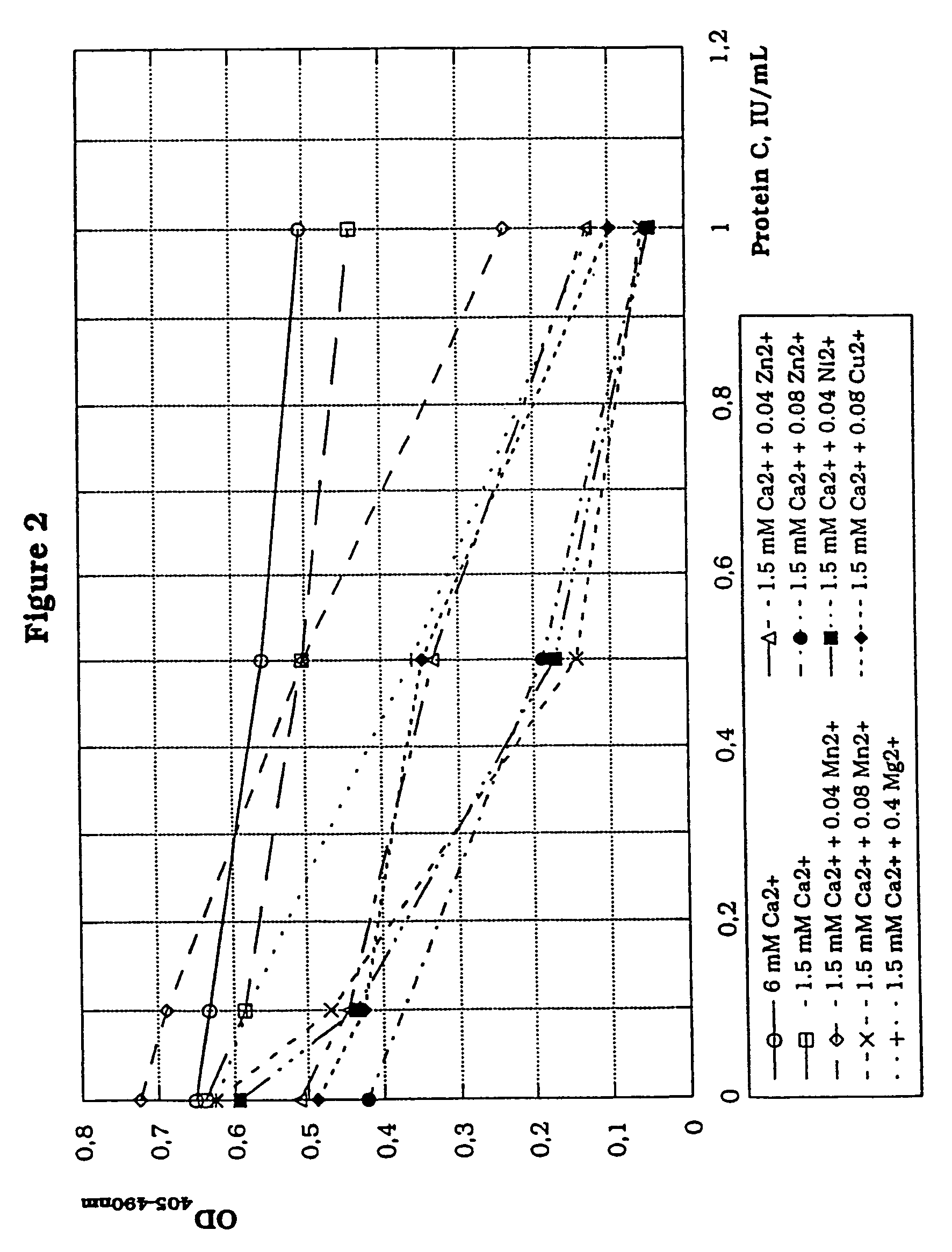
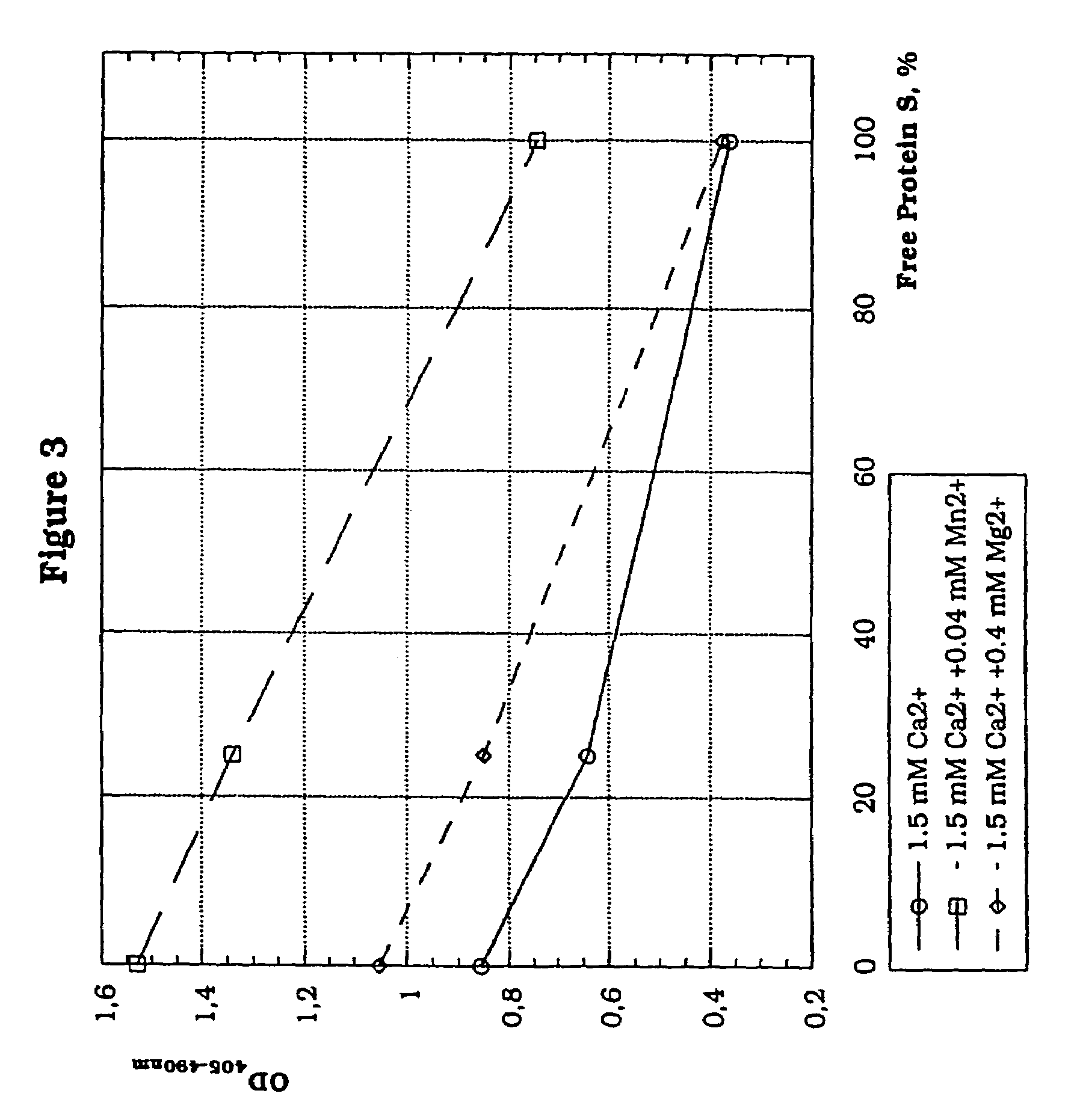
In vitro methods for qualitative screening and / or quantitative determination of the functional activity of components of the Protein C anticoagulant pathway of blood coagulation are described. The methods entail measuring the conversion rate of a substrate by an enzyme, the activity of which is related to the Protein C anticoagulant activity, in a blood sample of a human comprising coagulation factors and said substrate, after at least partial activation of coagulation through the intrinsic, extrinsic or common pathway and triggering coagulation by adding calcium ions; and comparing said conversion rate with the conversion rate of a normal human blood sample determined in the same way. The methods include the addition of additional metal ions to the sample to enhance activity, sensitivity and resolution. Kits and reagents for use in the methods are also provided.
Owner:INSTR LAB
Materials and methods for treating coagulation disorders
InactiveUS20030199573A1Safe and effective anticoagulant treatmentImprove the quality of lifeBiocideOrganic chemistryWarfarinPharmaceutical Substances
This invention is drawn to compounds which are more easily metabolized by the metabolic drug detoxification systems. Particularly, warfarin analogs which have been designed to include esters within the structure of the compounds are taught. The invention teaches methods of reducing the toxicity of drugs comprising the introduction of ester groups into drugs during the synthesis of the drug. This invention is also drawn to methods of treating coagulation disorders comprising the administration of compounds which have been designed to be metabolized by serum or intracellular hydrolases and esterases. Pharmaceutical compositions of the ester containing warfarin, analogs are also taught.
Owner:ARMETHEON INC
Exosite-directed thrombin inhibitors
InactiveUS20100267638A1Inhibiting generation of thrombinPeptide/protein ingredientsAnimals/human peptidesBlood coagulationsEnzyme inhibitor
Disclosed are an amino acid sequence of the human blood clotting factor Va, peptides containing such sequence, and additional peptides of interest that significantly inhibit thrombin generation. Also disclosed are pharmaceutical compositions containing these peptides and related therapeutic methods for inhibiting thrombin generation and treating blood coagulation disorders.
Owner:CLEVELAND STATE UNIVERSITY
Method for treating blood coagulation disorders
Provided is a method for treatment of blood coagulation disorders. The method comprises administering to an individual compositions comprising lipidic particles comprising phopatidylcholine, phosphatidylinositol and cholesterol. One or more peptides, polypeptides or proteins involved in the blood coagulation cascade are associated with the lipidic particles.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Improved preparations of adult liver progenitor cells
Owner:UNIVERSITE CATHOLIQUE DE LOUVAIN
Truncated von Willebrand factor polypeptides for treating hemophilia
ActiveUS10688157B2Large structureIncreased catabolismFactor VIIPeptide/protein ingredientsFactor VIII vWFHemophilias
The invention relates to a polypeptide comprising a truncated von Willebrand Factor (VWF) for use in the treatment of a blood coagulation disorder, wherein the polypeptide carries a half-life extending moiety and is administered in molar excess over Factor VIII and / or endogenous VWF.
Owner:CSL BEHRING LENGNAU AG
Bombyx mori extract and extraction method and application thereof
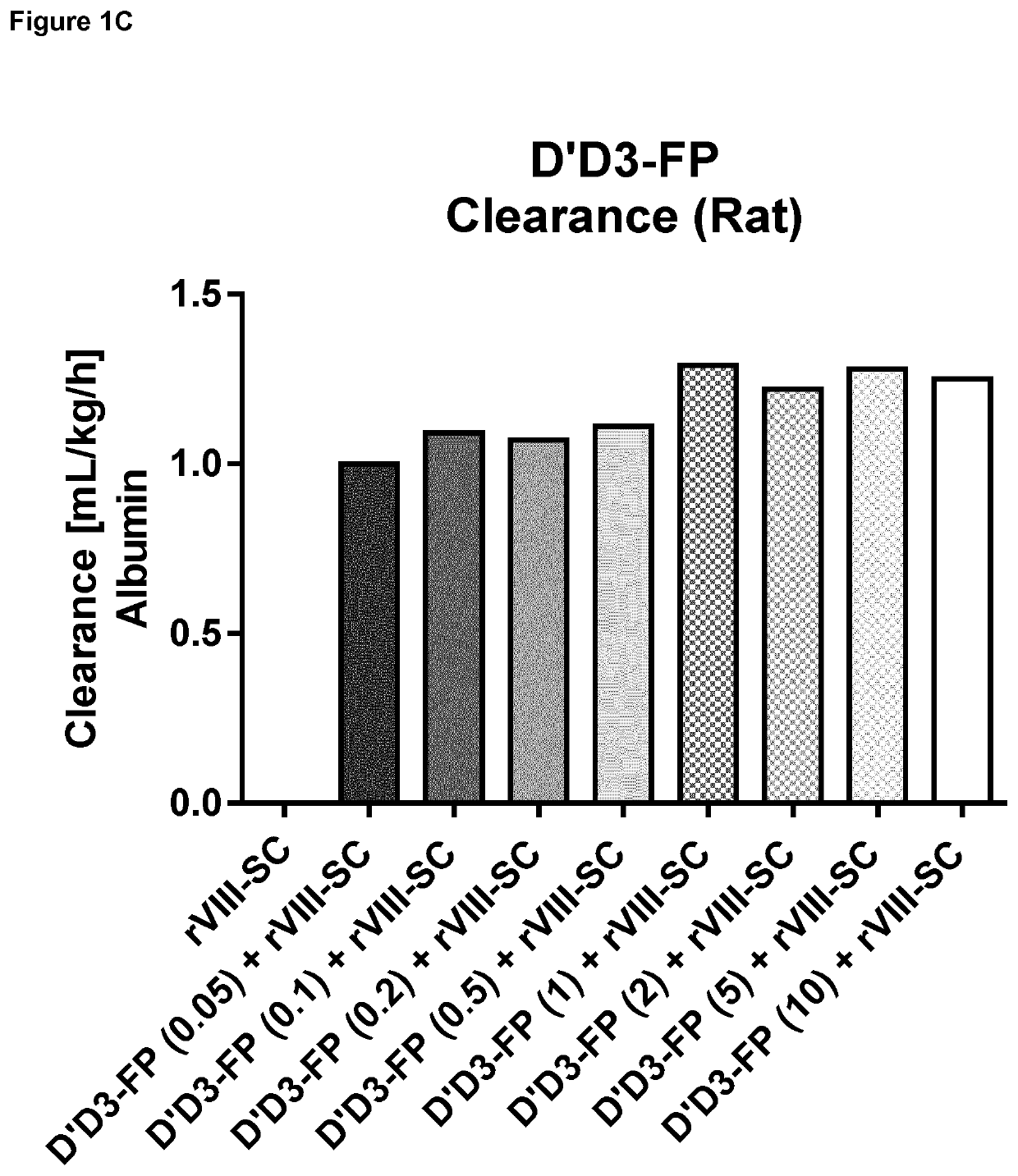
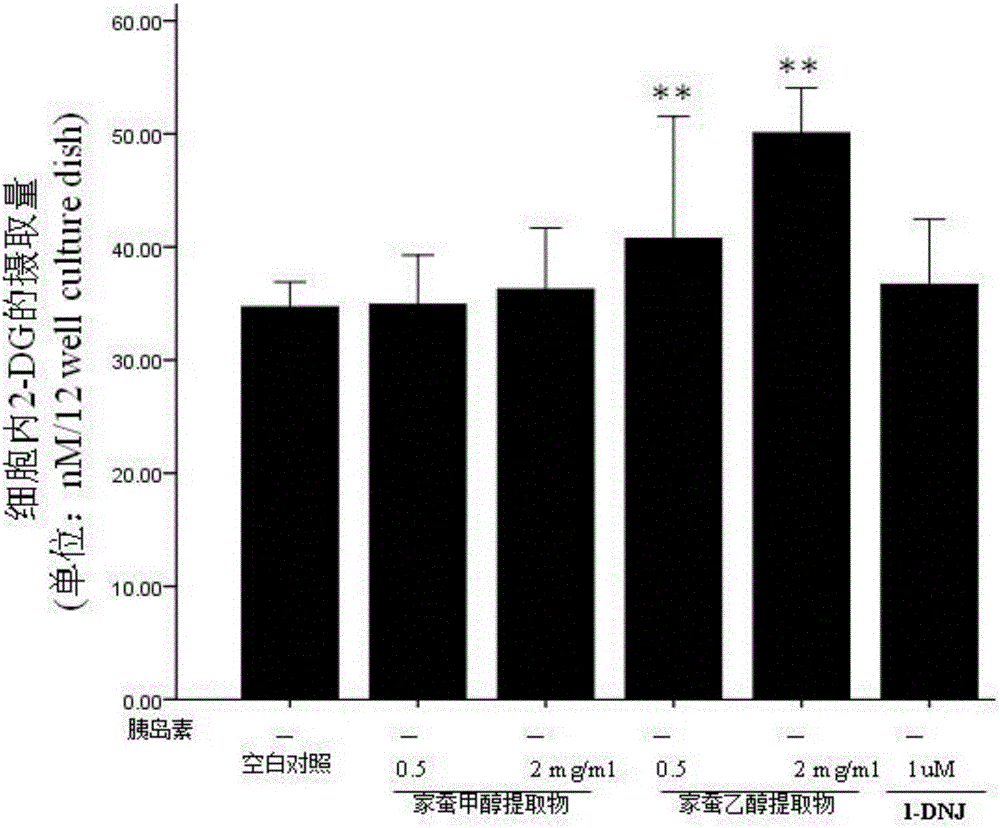
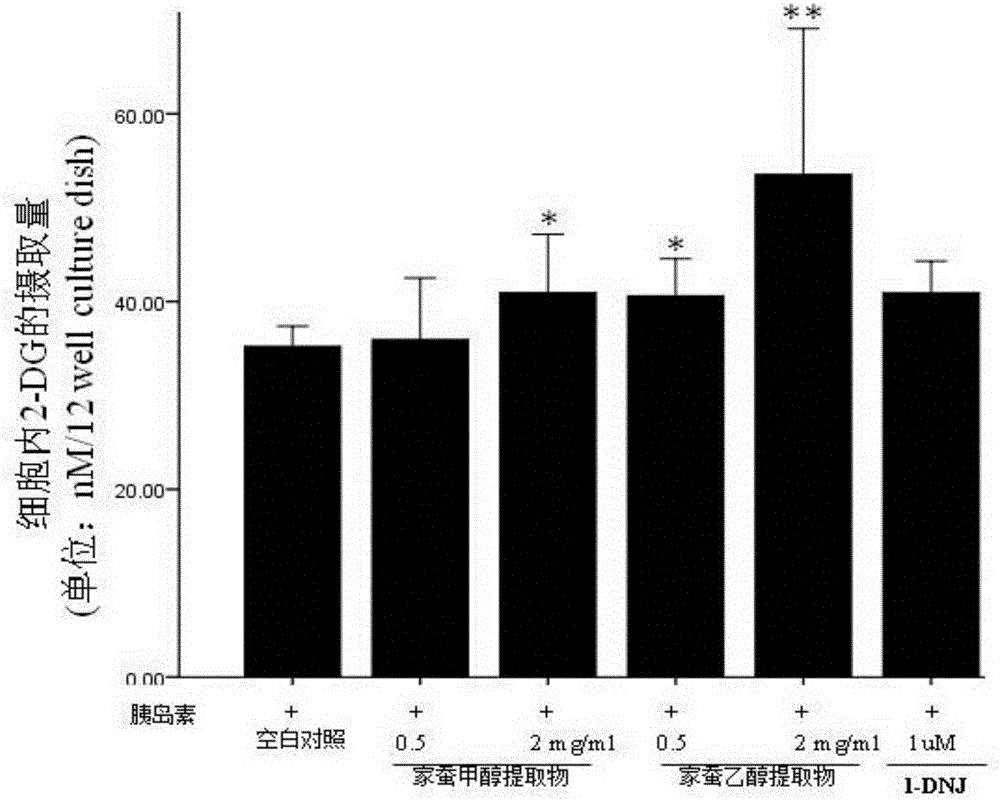
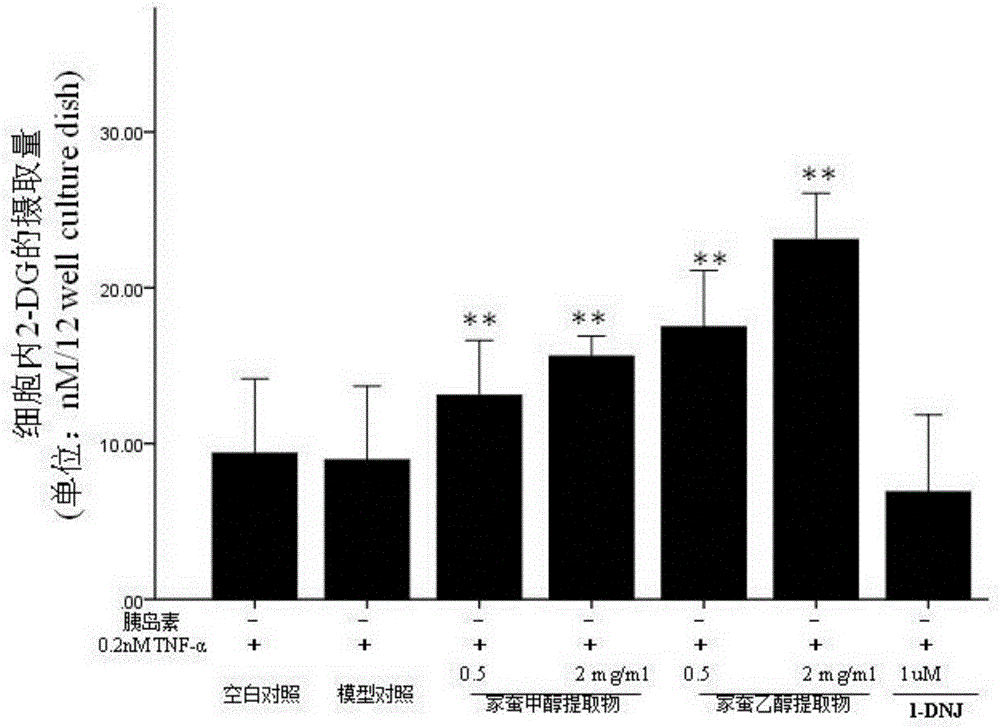
InactiveCN105769923AHigh activityImprove the immunityAnthropod material medical ingredientsMetabolism disorderDyslipidemiaFreeze-drying
The invention relates to a bombyx mori extract, in particular to the bombyx mori extract and an extraction method and application thereof. The extraction method includes the steps that 5-old bombyx mori is taken and prepared into bombyx mori freeze-dried powder after freeze drying and smashing, after an alcohol-water solution is added, soaking, ultrasonic treatment and filtering are conducted, and filtrate A is obtained; 2, an alcohol-water solution is added to sediment obtained in the step 1, soaking, ultrasonic treatment and filtering are conducted, and filtrate B is obtained; 3, the filtrate A and the filtrate B are combined, vacuum concentration and drying are conducted, and then the bombyx mori extract is obtained. The bombyx mori extract can be applied to relieving insulin resistance and enhancing cell glucose uptake. On the aspect of relieving insulin resistance, the bombyx mori extract is applied to preparing medicine for treating or relieving insulin resistance syndromes and preparing medicine for treating type 2 diabetes, obesity, hypertension, dyslipidemia, polycystic ovarian syndromes, hyper blood-coagulation disorder, microalbuminuria and other diseases. On the aspect of enhancing cell glucose uptake, the bombyx mori extract is applied to preparing medicine for treating or relieving type 1 diabetes.
Owner:厦门市医药研究所
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com