Truncated von willebrand factor polypeptides for treating hemophilia
A technology for factors and uses, applied in the field of improving the treatment of coagulation disorders, can solve the problem of not prolonging the survival of FVIII, and achieve the effect of reducing the frequency of administration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0234] 【Preparation of polypeptide】
[0235] Nucleic acids encoding polypeptides of the present invention can be prepared according to methods known in the art. Based on the cDNA sequence of VWF (SEQ ID NO: 3), recombinant DNA encoding the above-mentioned truncated VWF construct or polypeptide of the present invention can be designed and produced.
[0236] Even if the polypeptide secreted by the host cell does not comprise amino acids 1 to 763 of VWF, it is preferable that the nucleic acid (eg, DNA) encoding the intracellular precursor of the polypeptide comprises a nucleotide sequence encoding the amino acid sequence. Has at least 95%, at least 96%, at least 97%, at least 98% or at least 99% sequence identity to amino acids 23-763 or preferably to amino acids 1-763 of SEQ ID NO:4. Most preferably, the nucleic acid (eg DNA) encoding the intracellular precursor of the polypeptide comprises a nucleotide sequence encoding amino acids 23-763 of SEQ ID NO:4 or amino acids 1-763 of...
Embodiment 1
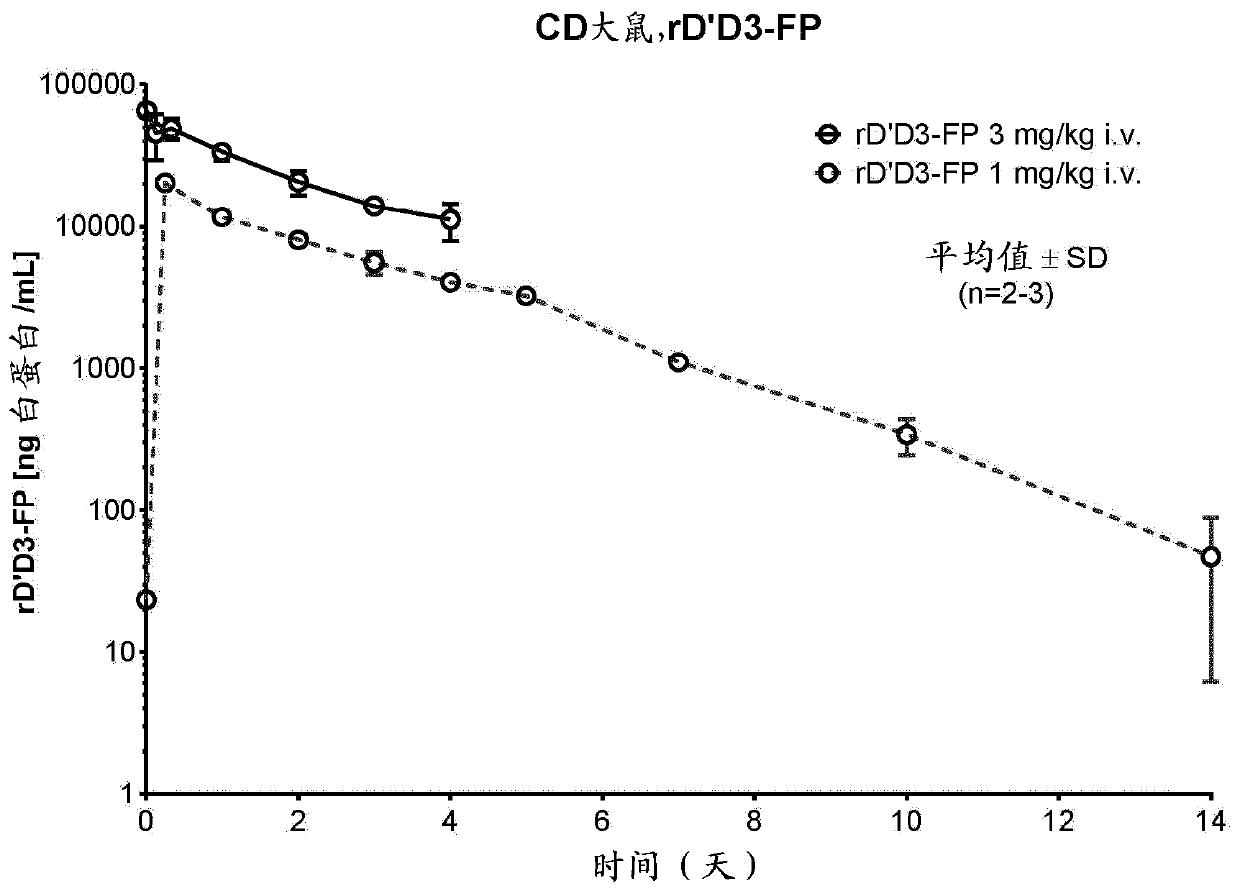
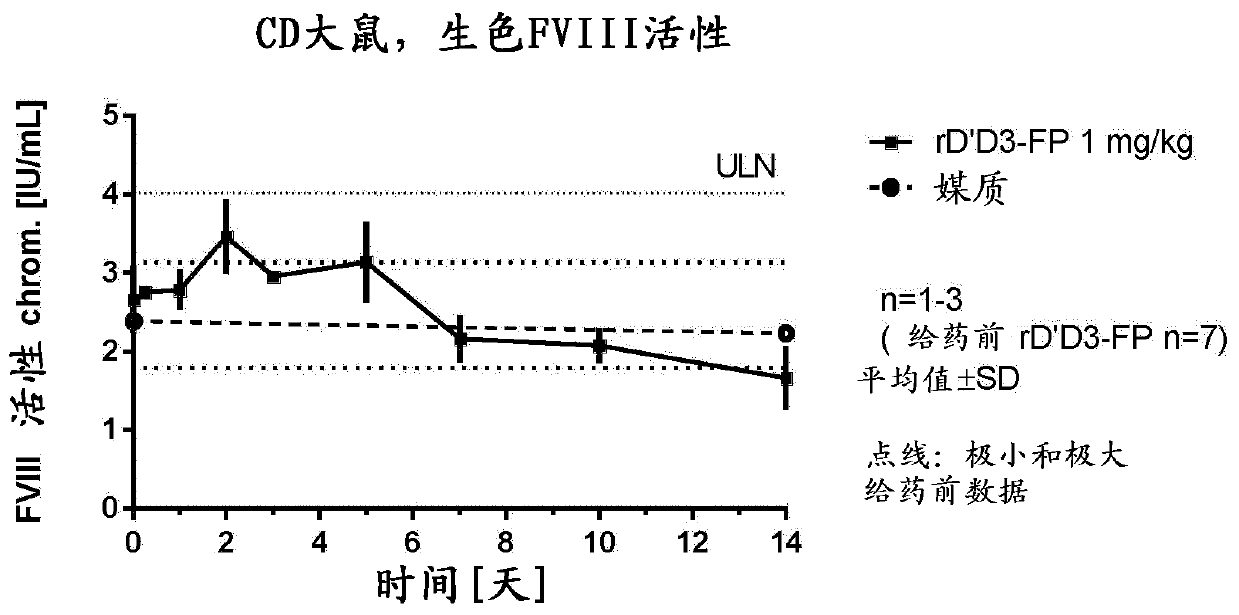
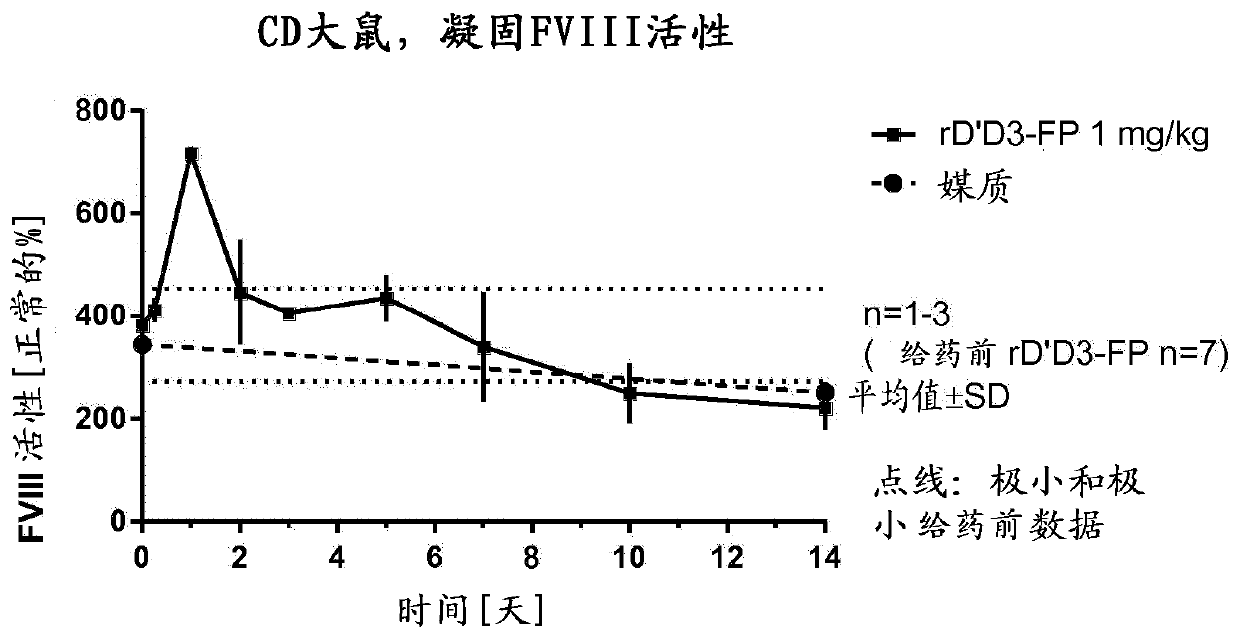
[0311] [Example 1: Analysis of FVIII levels in plasma after administration of rD'D3-FP]
[0312] Our aim was to characterize the effect of rD'D3-FP on endogenous FVIII levels, thereby generally supporting the treatment of mild to moderate or severe hemophilia A patients with low levels of VWF and functional endogenous FVIII or certain Types of von Willebrand disease. We studied this effect in different ways:
[0313] Models with normal endogenous FVIII and VWF levels, namely rats (Example 1.1), rabbits (Example 1.2) and monkeys (Example 1.3), were administered intravenously to study intravenous (iv ) Potential further increase of endogenous FVIII after administration of rD'D3-FP.
[0314] Models with low FVIII levels due to VWF deficiency, namely VWF ko rats (Example 1.4) and VWF ko mice (Example 1.5), were administered intravenously to study intravenous administration of rD'D3 in diseased subjects - Increased size of endogenous FVIII after FP.
[0315] VWF ko rats, a mode...
Embodiment 11
[0355] [Example 1.1: Effect of intravenous treatment with rD'D3-FP on physiological endogenous FVIII levels in rats]
[0356] 【animal】
[0357] Female Crl:CD (Sprague Dawley) rats weighing in the range of 200-294 g were bred at Charles River Laboratories (Sulzfeld, Germany). Indoors, animals were maintained under standard cage conditions, ie, 12-24°C, 12h / 12h light-dark cycle. with standard mouse and rat diet (Ssniff Soest, Germany) fed the animals ad libitum. Free supply of tap water. Animal husbandry and research procedures complied with German animal welfare law and EU regulations.
[0358] For the 1 mg / kg group, the group size was n=9, divided into 3 groups except for the control (n=3 animals only). The group size of the 1 mg / kg group was n=6, divided into 2 groups. Therefore, n=3 animals per time point were always used.
[0359] 【Experiment Details】
[0360] Test articles were administered intravenously by a single injection into the lateral tail vein of rats (n=...
PUM
| Property | Measurement | Unit |
|---|---|---|
| elongation | aaaaa | aaaaa |
| elongation | aaaaa | aaaaa |
| elongation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com