Methods and devices for detection of anticoagulants in plasma and whole blood
A microfluidic device, a technology for blood clots, applied in the field of assessing coagulation in blood samples, detecting anticoagulants or abnormal coagulation, and solving problems such as limited functional information
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
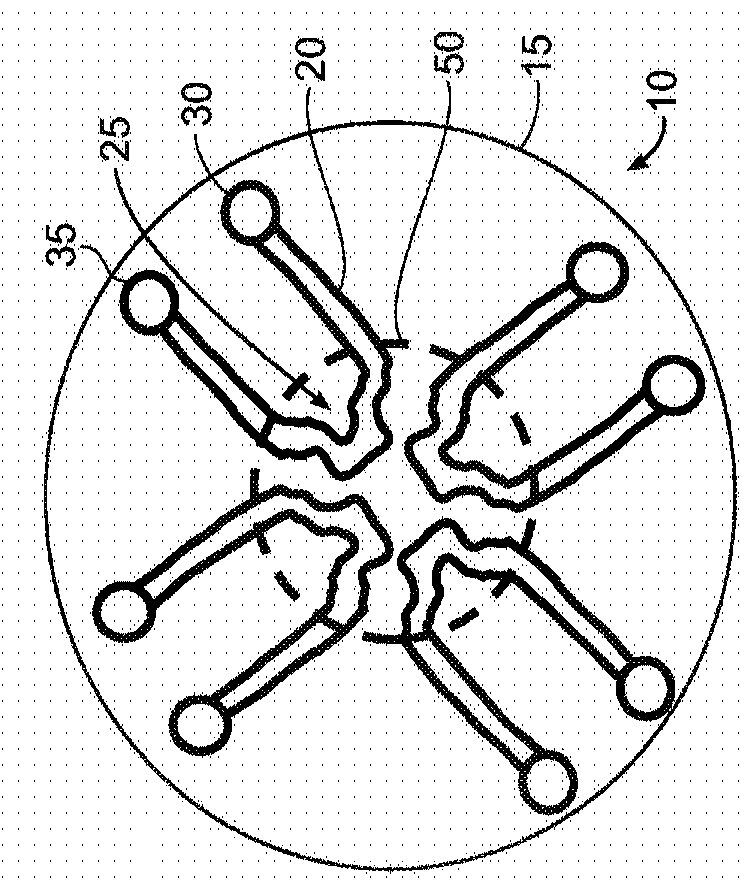
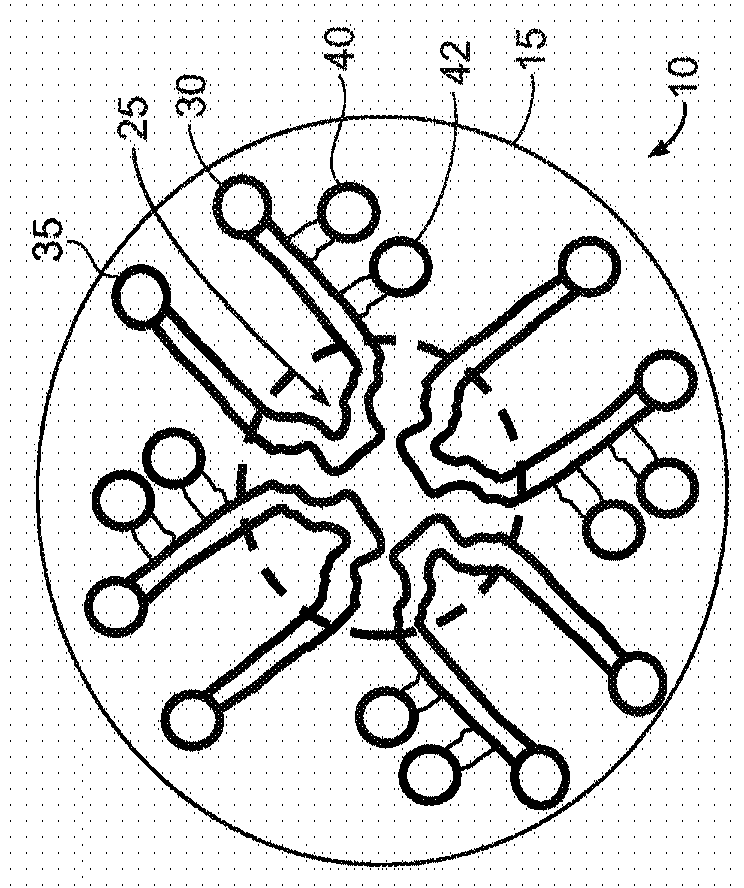
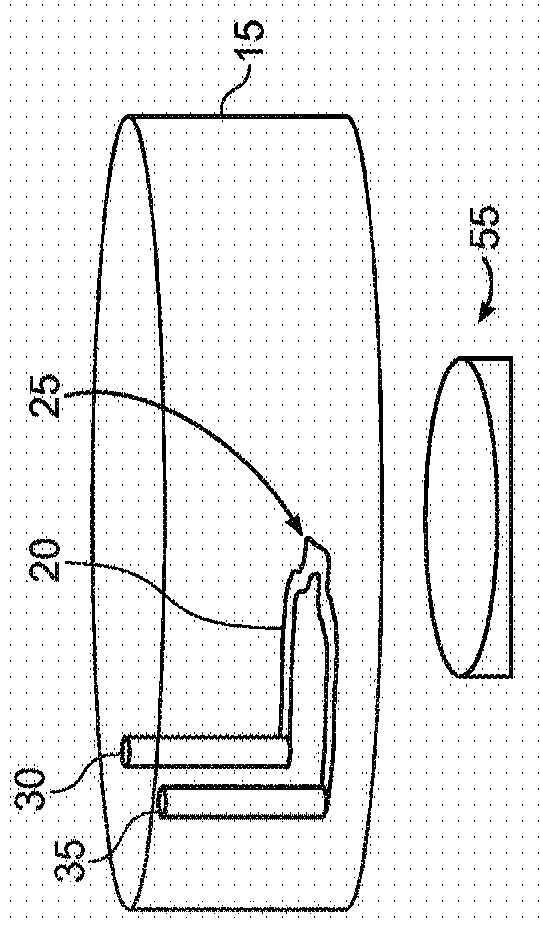
[0148] Figures 1A to 1D is a schematic illustration of a microfluidic device layout according to some exemplary embodiments of the invention.
[0149] Figure 1A is a top view of a circular layout (which can also be any symmetrical polygon with a center point) of a microfluidic device 10 with one or more continuous microfluidic channels formed in a substrate 15 (eg, microchannels) 20, each channel is connected to an inlet (input port) 30 and an outlet (output port) 35. A portion of the channel (eg, the center of the channel) may have a unique shape (eg, clot formation / localization region 25 ) to achieve flow separation or perturbation, or stagnation of sample flow to promote clot formation. Depending on the particular assay used, there may be two or more of these microfluidic channels in the single device. This design may allow multiple samples to be evaluated simultaneously, eg three or more samples, eg up to 10 samples or more than 10 samples. Typically, each sample (or...
Embodiment 2
[0158] A general protocol for performing an assay according to one embodiment of the invention is as follows:
[0159] a) Add sample, agonist, + / - calcium, + / - clot detection reagent together
[0160] i. The final concentration of calcium is 0.2mM (This concentration is especially suitable for use with 3.2% buffered sodium citrate. If using additional anticoagulants, the concentration of calcium may be different from 0.2mM.)
[0161] ii. Blood clot detection reagents can include fluorescently labeled fibrinogen, magnets, beads (can be fluorescent or colored)
[0162] b) Loading into a microfluidic device
[0163] i. For an example of entering the load configuration and order, see e.g. Figures 1A to 1D , 2A and 2B
[0164] c) Temperature control
[0165] i. room temperature
[0166] ii. Can rise to 37°C (body temperature)
[0167] (The body temperature is usually 37°C, but the temperature of the assay run can be changed according to the actual temperature of the patient....
Embodiment 3
[0171] Figures 3A to 3C Blood clot detection using plasma and fluorescently labeled fibrinogen is shown with a microfluidic device 310 having four channels 320 and a clot formation / localization region 225 according to an exemplary embodiment. The microfluidic device is similar to Figure 2A and Figure 2B The device shown in , differs in that all clot formation regions 325 have the same shape. Each clot formation / localization region 325 contains protrusions for perturbing sample flow. In this example, as Figure 3A As shown in , the protrusions are usually triangular in shape. Two sides of the protrusion are straight and one side is concave. Each clot formation region 325 redirected the flow four times, including two 90 degree changes of direction.
[0172] In one example, the blood clot detection process may include the following procedural steps:
[0173] a) Plasma samples were premixed to contain: 6 μL plasma + 0.6 μL agonist (10% sample volume) + 0.6 μL calcium (st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com