Biomarker for prognosis or recurrence early warning evaluation of acute ischemic stroke and application of biomarker
A technology of ischemic stroke and biomarkers, applied in the field of biomarkers, can solve the problems of inability to meet clinical needs, high prognosis prediction value of AIS, and achieve the effect of improving clinical services
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] Example 1: Collection of clinical data of acute ischemic stroke patients and healthy control groups
[0076] This study included inpatients in Beijing Tiantan Hospital Affiliated to Capital Medical University from October 2018 to November 2019 and patients in the multicenter China National Stroke Registry Study-Ⅲ (CNSR-3). Inclusion criteria for patients with acute ischemic stroke: (1) The age of onset is between 18 and 80; (2) Patients with acute cerebral infarction are clearly diagnosed according to the patient's clinical and imaging data; (3) The cause of the onset is aortic Atherosclerotic; (4) There is symptomatic intracranial or extracranial arterial stenosis (decrease in arterial lumen diameter ≥ 50%). The included clinical information includes: general basic information and clinical characteristics of patients, high risk factors for aortic atherosclerosis (hypertension, diabetes, coronary heart disease, hyperlipidemia, etc.), and acute phase treatment plan. Fol...
Embodiment 2
[0077] Example 2: Collection and storage of samples from patients with acute ischemic stroke and healthy controls
[0078] 1. Collection of plasma
[0079] Collect peripheral blood in purple tubes containing anticoagulant EDTA or heparin, and centrifuge within 30 minutes after sample collection, at 3000 rpm for 10 minutes, at 2-8°C. The upper plasma layer was collected and stored in batches at -80°C. Avoid repeated freezing and thawing of samples. Note: Samples should be centrifuged sufficiently to avoid hemolysis or the presence of particles.
[0080] 2. Extraction of exosomes in peripheral plasma
[0081] ExoQuick Extracellular Vesicle Isolation Kit (Catalog No.: EQULTRA-20A-1) for serum and plasma was used for the detection.
[0082] 2.1 Extraction
[0083] 1) Take out the sample, centrifuge at 3000g for 15min to get the supernatant.
[0084] 2) If there are still cell debris remaining, centrifuge the supernatant again at 12,000g for 10min, and transfer the supernatan...
Embodiment 3
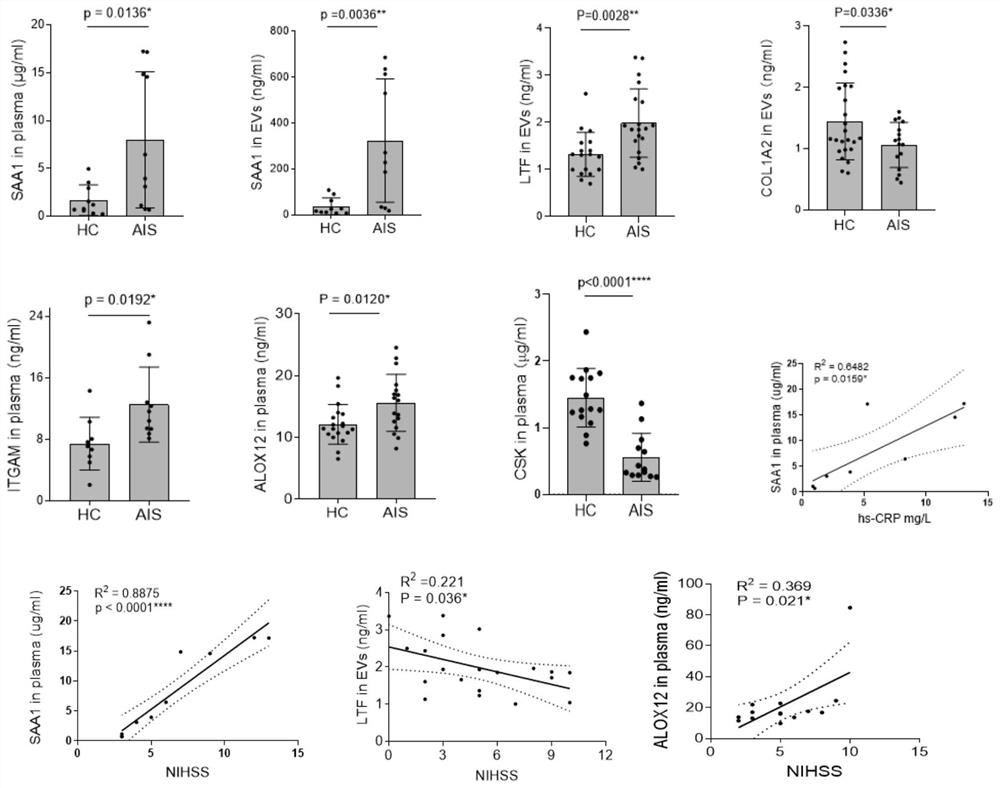
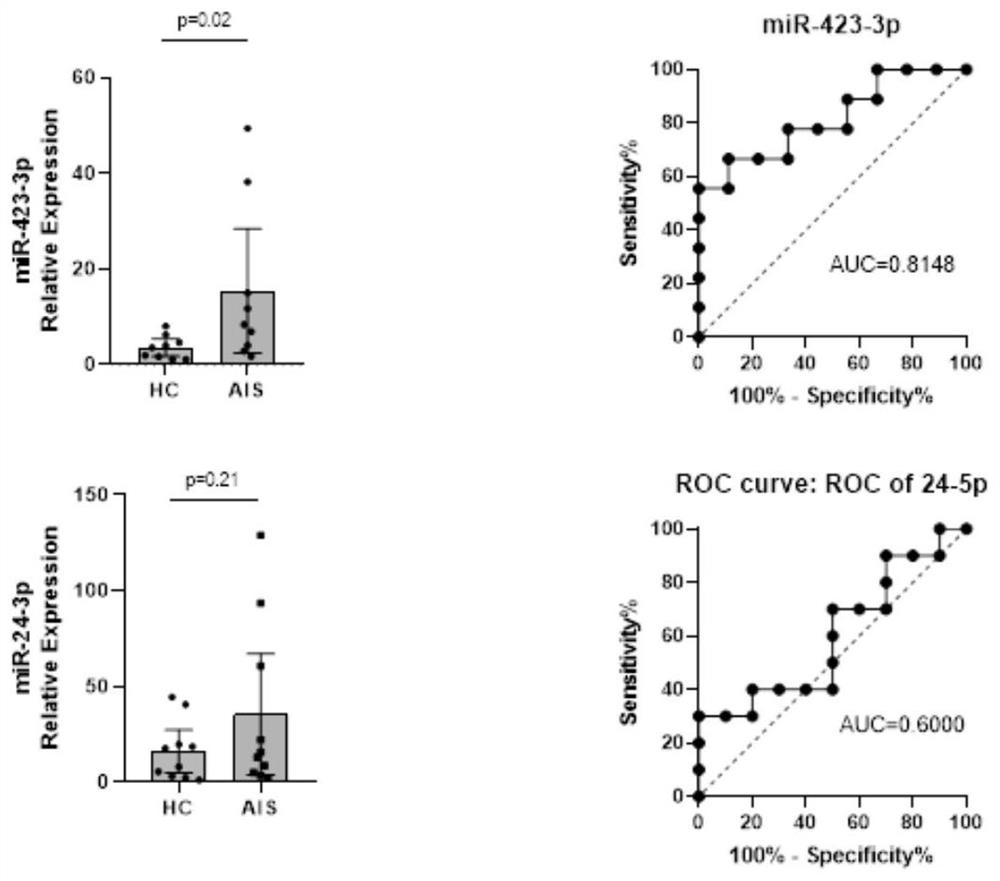
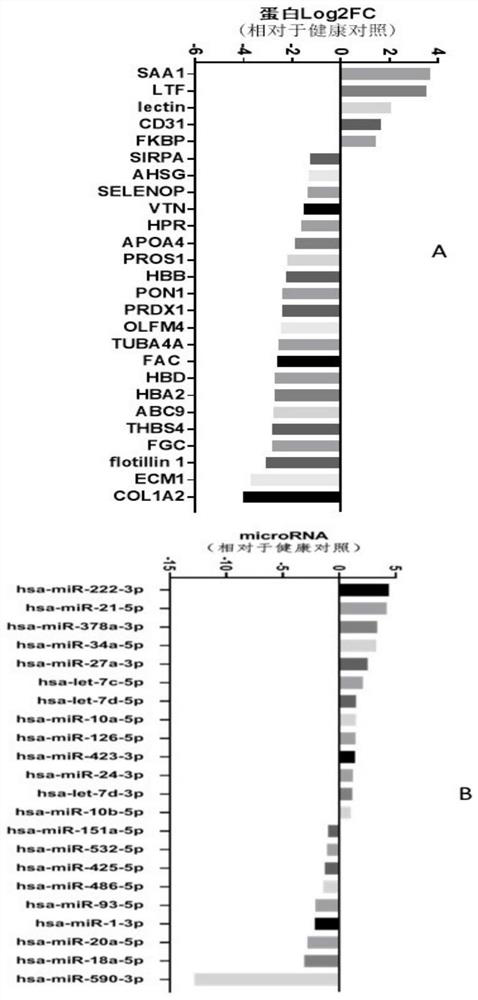
[0111] Example 3: Analysis of genes and proteomics, screening out differentially expressed proteins, and obtaining the detection combination of biomarkers for recurrence and prognosis assessment
[0112] 1. This project uses the next generation of non-labeled quantitative proteomics technology to complete the analysis. In the data-independent acquisition (Data independent acquisition, DIA) mode, it can provide unparalleled proteome coverage, while achieving accurate, Highly reproducible quantification. The DIA process provides an ideal platform for qualitative analysis of differentially expressed proteomes or quantitative proteomes of massive samples. The DIA process is based on three necessary steps:
[0113] 1) Construction of a spectral library: The spectral library collects all detectable non-redundant high-quality peptide information (MS / MS spectra) of the sample, which is used as a peptide identification template for subsequent data analysis. It includes fragment ion i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com