Method for manufacturing high purified factor ó¨
A blood coagulation factor and factor technology, which is applied in the field of preparation of human coagulation factor IX, can solve problems such as inability to use, filter clogging, and inability to rule out side effects
Active Publication Date: 2008-10-22
THE GREEN CROSS CORP
View PDF0 Cites 7 Cited by
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
And, there is also a more effective nanofiltration method that utilizes nanofiltration (nanofilter, 20nm), but when utilizing this nanofiltration, it is necessary to carry out a high-purity concentration process to the blood coagulation factor in advance, so as to prevent it from occurring in the use of non-high-purity When the prepared coagulation factor is subjected to nanofiltration, the filter is clogged and cannot be used
Although factor IX can be selectively isolated by monoclonal antibody (monoclonal antibody), since animal cells are required to produce antibodies, virus infection derived from animal cells cannot be ruled out
In addition, side effects caused by immunoglobulin (IgG) eluted with antibodies during the chromatography process cannot be ruled out
Method used
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
View moreImage
Smart Image Click on the blue labels to locate them in the text.
Smart ImageViewing Examples
Examples
Experimental program
Comparison scheme
Effect test
Embodiment 1
Embodiment 2
Embodiment 3
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More PUM
| Property | Measurement | Unit |
|---|---|---|
| Specific activity | aaaaa | aaaaa |
Login to View More
Abstract
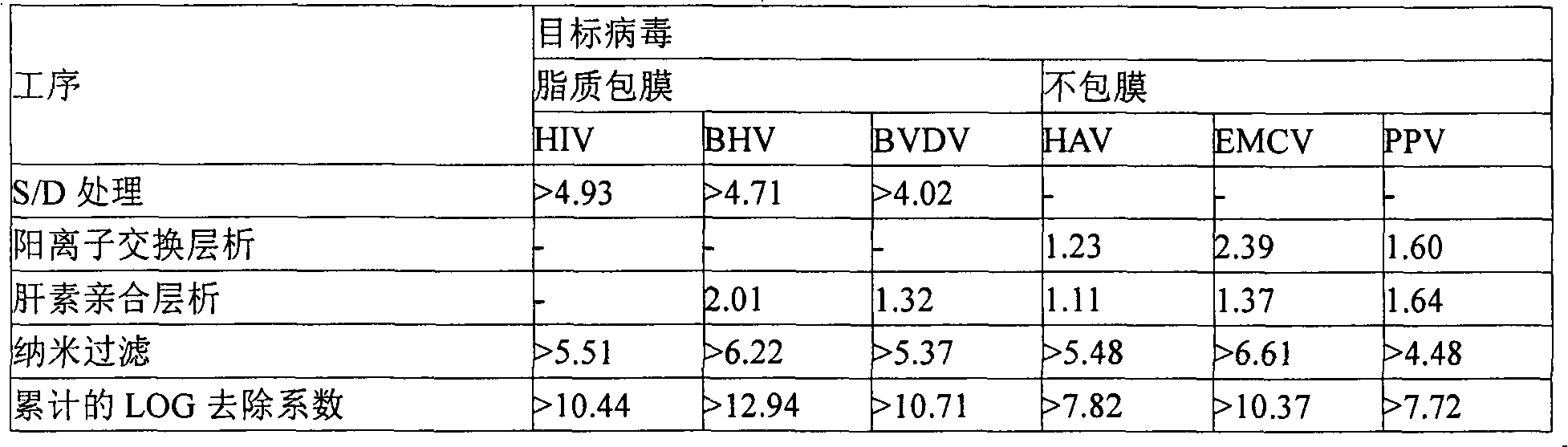
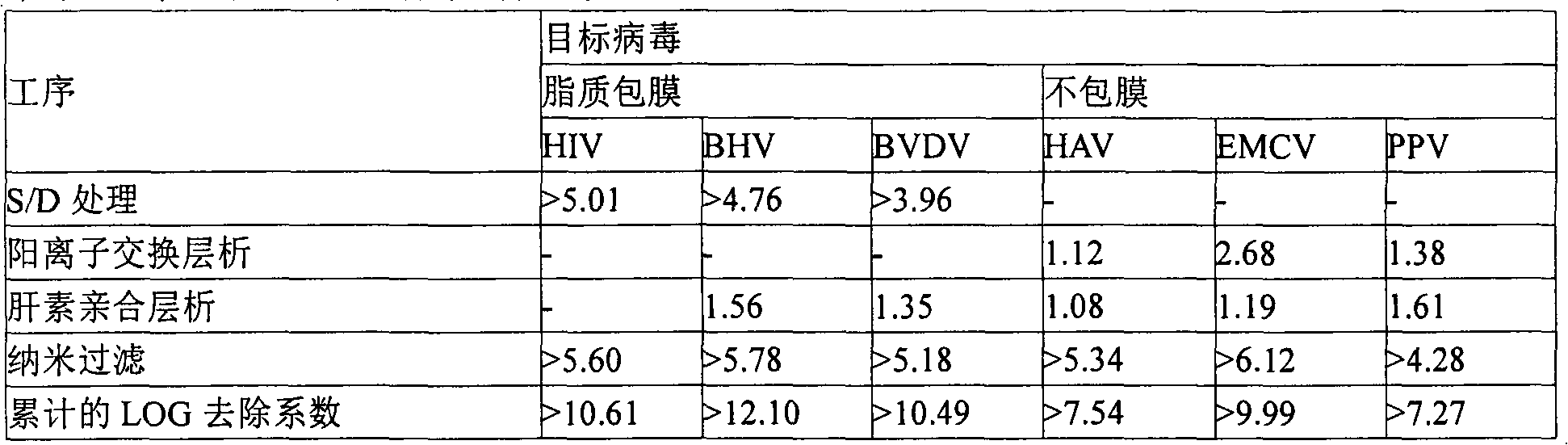
The present invention discloses a preparation method of a highly purified human blood coagulation factor IX. The human blood coagulation factor IX is prepared by performing anion exchange chromatography, cation exchange chromatography, heparin affinity chromatography to the material containing human blood coagulation factor IX (taken from human blood serum or recombined cell culture medium), which includes the period of passivating virus through S / D treatment (Solvent / Detergent treatment) or removing virus through nanofiltration. The highly purified safe coagulation factor IX preparation having a specific activity of above 150 IU / mg, with substantially undoped proteins can be prepared by the preparation method of the invention.
Description
A kind of preparation method of high-purity human coagulation factor IX technical field The present invention relates to a preparation method of high-purity human coagulation factor IX, in particular to a method by performing ion-exchange chromatography and affinity layer on a substance containing coagulation factor IX (taken from human plasma or recombined cell culture medium). In the stage of virus inactivation or virus removal by analysis, the safe high-purity coagulation factor IX with almost no adulterant protein and specific activity (specific activity) above 150IU / mg can be produced. Background technique Human coagulation factor IX is an indispensable glycoprotein in the blood coagulation process. If there is no coagulation factor IX or insufficient coagulation factor IX in the blood due to genetic factors or pathological factors, the coagulation process in the blood will be incomplete. , causing hemophilia B. Hemophilia B mainly occurs through heredity. One in 30...
Claims
the structure of the environmentally friendly knitted fabric provided by the present invention; figure 2 Flow chart of the yarn wrapping machine for environmentally friendly knitted fabrics and storage devices; image 3 Is the parameter map of the yarn covering machine
Login to View More Application Information
Patent Timeline
 Login to View More
Login to View More IPC IPC(8): C07K1/14
CPCC12Y304/21022C12N9/644C07K1/14
Inventor 姜镛崔龙云孙基桓李成来成学模金基镕许在旭
Owner THE GREEN CROSS CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com