A method for simultaneously separating and purifying blood coagulation factors ix, x and ⅶ from human plasma
A technology for the simultaneous separation of coagulation factors, applied in chemical instruments and methods, coagulation/fibrinolytic factors, peptide preparation methods, etc., can solve the problems of long time-consuming and cumbersome steps, and achieve short time-consuming, simple operation, and realization Take full advantage of the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
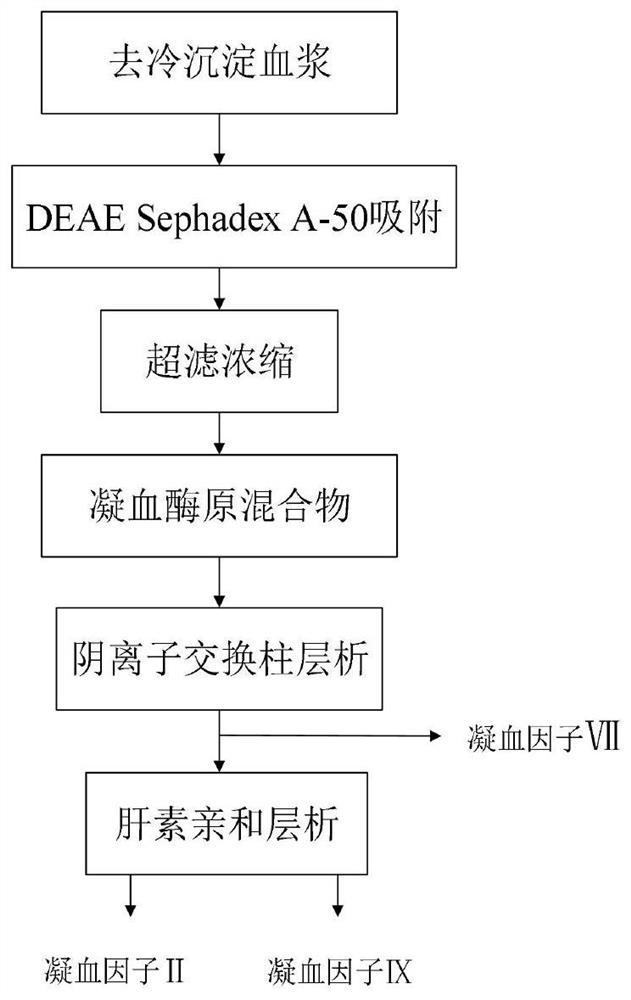
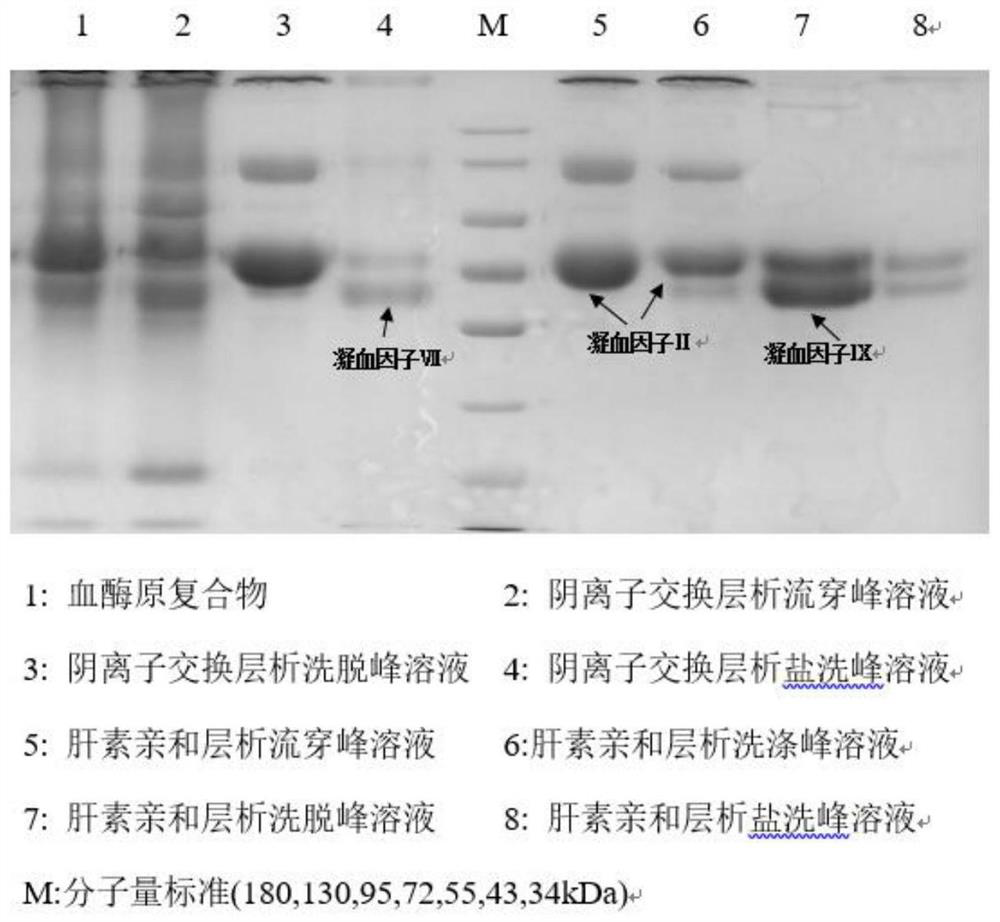
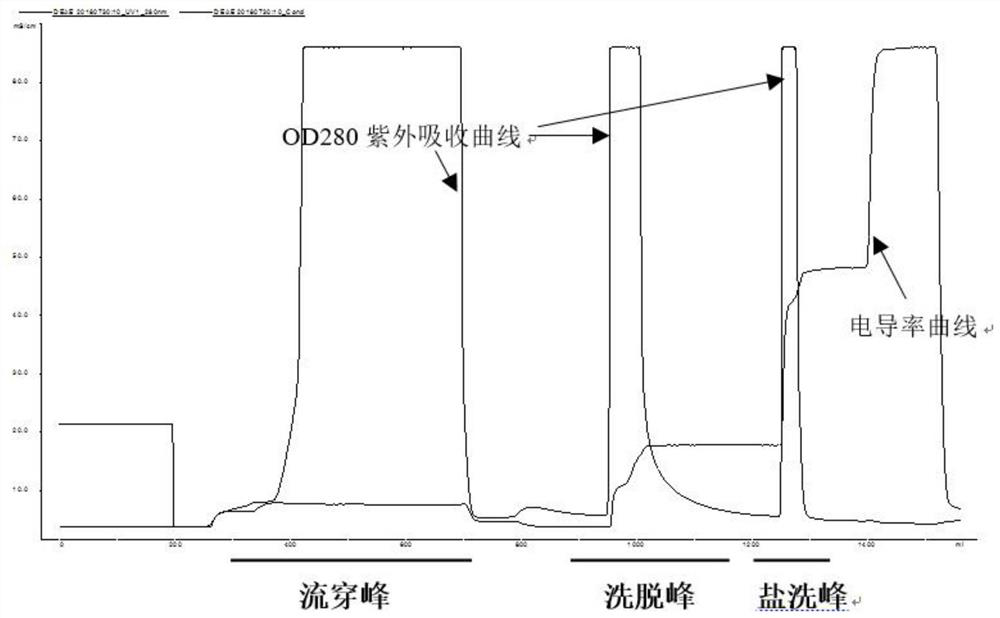
[0036] The technical process of a method for simultaneously separating and purifying blood coagulation factors IX, X and VII from human plasma according to the present invention is as follows: figure 1 , the specific steps include:
[0037] 1. Preparation of prothrombin complex
[0038] S101. Plasma centrifugation to remove cryoprecipitate. Pre-cool the centrifuge in advance. Melt the plasma and centrifuge at 2.0°C to separate and remove the cryoprecipitate. After collection and separation, the plasma is heated to 6.0°C and filtered step by step with 10.0um and 1.0um filter elements until adsorption Can.
[0039] S102. Plasma adsorption and gel washing The adsorption gel medium is DEAE-Sephadex-A50, and the gel balance solution is 10mM citric acid-sodium citrate buffer containing 75mM sodium chloride, pH 7.0. Pump the adsorption equilibrium liquid with 2 times the volume of the gel into the adsorption tank, stir and mix evenly, discard it by suction filtration, and repeat un...
Embodiment 2
[0063] 1. Preparation of prothrombin complex
[0064] S101 Plasma centrifugation to remove cryoprecipitate Pre-cool the centrifuge in advance, melt the plasma and centrifuge at 0°C to separate and remove cryoprecipitate. After collection and separation, the plasma is heated to 4.0°C and filtered to the adsorption tank with a 10μm filter element.
[0065] S102 plasma adsorption and gel washing The adsorption gel medium is DEAE-Sephadex-A50, and the gel balance solution is 15mM citric acid-sodium citrate buffer containing 50mM sodium chloride, pH 6.0. Pump the adsorption balance liquid with 1 times the volume of the gel into the adsorption tank, stir and mix well, discard it by suction filtration, and repeat until the pH value of the suction filtrate is 6.0. After mixing the plasma and the gel in a certain ratio, keep it at 8.0°C, stir and absorb for 20 minutes, and filter the plasma to collect the gel.
[0066] S103 Gel equilibration, washing, and elution The gel washing solut...
Embodiment 3
[0085] 1. Preparation of prothrombin complex
[0086] S101 Plasma centrifugation to remove cryoprecipitate Pre-cool the centrifuge in advance, centrifuge the plasma at 8°C after melting, separate and remove cryoprecipitate, collect and separate and filter to the adsorption tank with a 0.2μm filter element.
[0087] S102 plasma adsorption and gel washing The adsorption gel medium is DEAE-Sephadex-A50, and the gel balance solution is 5mM citric acid-sodium citrate buffer containing 100mM sodium chloride, pH 8.0. Pump 1.5 times the gel volume of the adsorption equilibrium liquid into the adsorption tank, stir and mix well, discard by suction filtration, and repeat until the pH value of the suction filtrate is 8.0. After mixing the plasma and the gel in a certain ratio, stir and absorb for 60 minutes, and collect the gel by suctioning the plasma.
[0088] S103 Gel equilibration, washing, and elution The gel washing solution is 100mM sodium chloride, 5mM citric acid-sodium citrate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com