Recombinant adeno-associated virus vectors and methods for treating or preventing hemophilia B
A virus particle, construct technology, applied in the field of gene therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0192] Embodiment 1. Selection of highly active FIX mutants
[0193] In the early clinical trials of FIX gene therapy, the wild-type FIX protein sequence was selected, and the codon optimization of the FIX coding sequence was not performed, and the steady-state maintenance level of FIX activity was low and the time was short.
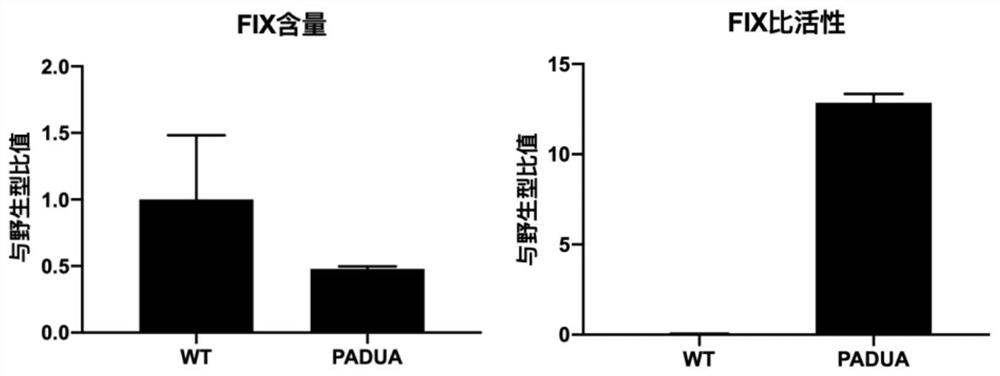
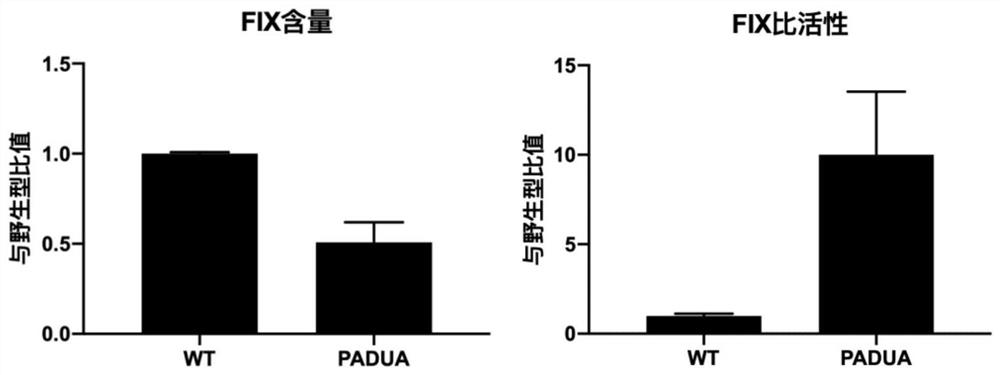
[0194] FIX PADUA is a naturally occurring FIX mutant with a single amino acid mutation (R338L) found in an Italian adolescent patient with venous thrombosis. Compared with wild-type FIX, the coagulation activity (FIX:C) can be increased by 5-10 times under the same expression level (FIX:Ag). Spark's SPK-9001, UniQure's AMT-061 and Freeline's FLT180a all selected highly active FIX PADUA variants as transgenes.
[0195] To confirm FIX PADUA Whether the variant does have improved expression levels and protein activity relative to wild-type FIX, the FIX WT 、FIX PADUA Ligated into scAAV vectors and ssAAV vectors and used to transfect human liver tumo...
Embodiment 2
[0198] Screening of Regulatory Elements in Example 2.FIX Gene Expression Cassette
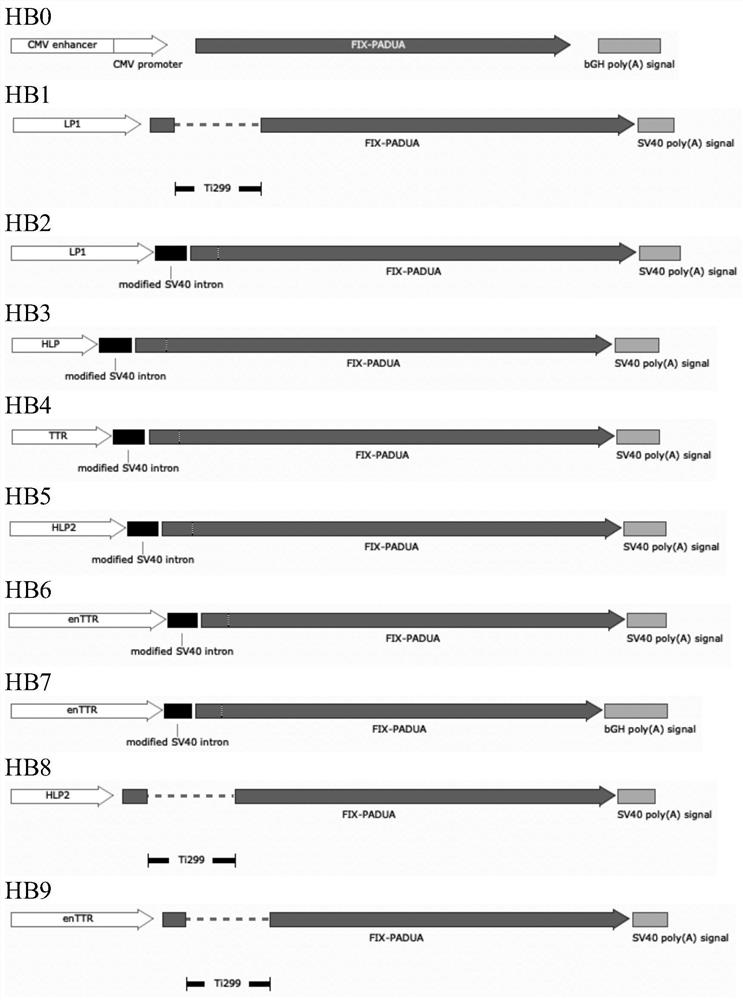
[0199] The inventors selected different regulatory elements to design and prepare 9 scAAV vector expression cassettes (HB1-HB9) and 8 ssAAV vector expression cassettes (HB10-HB17) ( image 3and Table 1), compared their expression efficiencies in human liver tumor cells HepG2 and Huh7, and expressed in CMV-FIX PADUA As a control (HB0). Considering the difference in the DNA loading capacity of scAAV and ssAAV, the FIX expression efficiency of scAAV and ssAAV vectors were compared separately.
[0200] Table 1. Combinations of regulatory elements in the constructs
[0201]
[0202]
[0203] The transfection conditions were the same as in Example 1. Cell culture supernatant was collected 72 hours after transfection for FIX ELISA detection (abcam, ab168546) and Biophen FIX activity detection (HYPHEN BioMed, 221802).
[0204] The HepG2 cell experiment results of the first batch of 7 scAAV ...
Embodiment 3
[0207] Example 3. Optimization of Coding Sequences
[0208] The inventor designed and synthesized 8 codon-optimized FIX PADUA The coding sequences are respectively named as FIX-PADUA-co1 to FIX-PADUA-co8, and the nucleotide sequences thereof are respectively shown in SEQ ID NOs: 16-23. Using these codon-optimized coding sequences, the corresponding recombinant vectors were constructed and the transduction experiments as described in previous examples were repeated in HepG2 and Huh7 cells.
[0209] Figure 10 The detection results of the expression level and activity of the above 8 different codon optimized sequences delivered by the HB9 vector in HepG2 cells are shown, and are compared with the FIX of SEQ ID NO:12 without codon optimization PADUA Coding sequences were compared. From Figure 10 The results show that FIX-PADUA-co8, FIX-PADUA-co5 and FIX-PADUA-co2 are the three codon-optimized sequences with the best performance, especially the performance of FIX-PADUA-co8 an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com