Coagulation factor ⅸ quality control product preparation method
A technology for coagulation factors and quality control products, applied in the field of preparation of clinical coagulation test preparations, can solve problems such as single level, high product price, inability to meet the needs of multiple activity levels, etc. good effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0015] In conjunction with embodiment, further set forth the present invention:
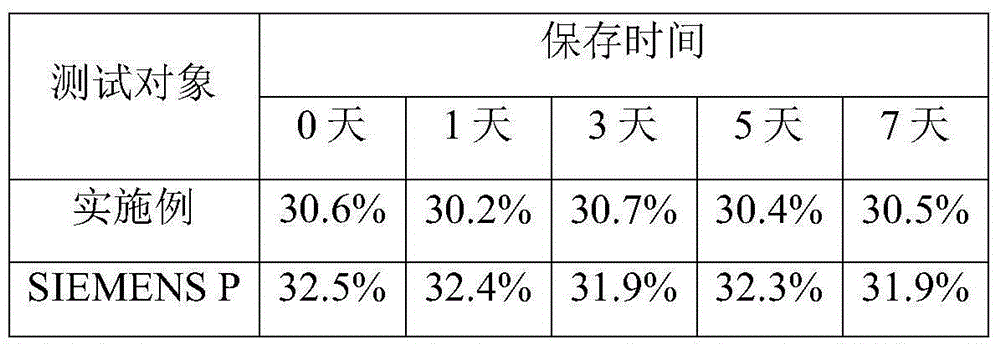
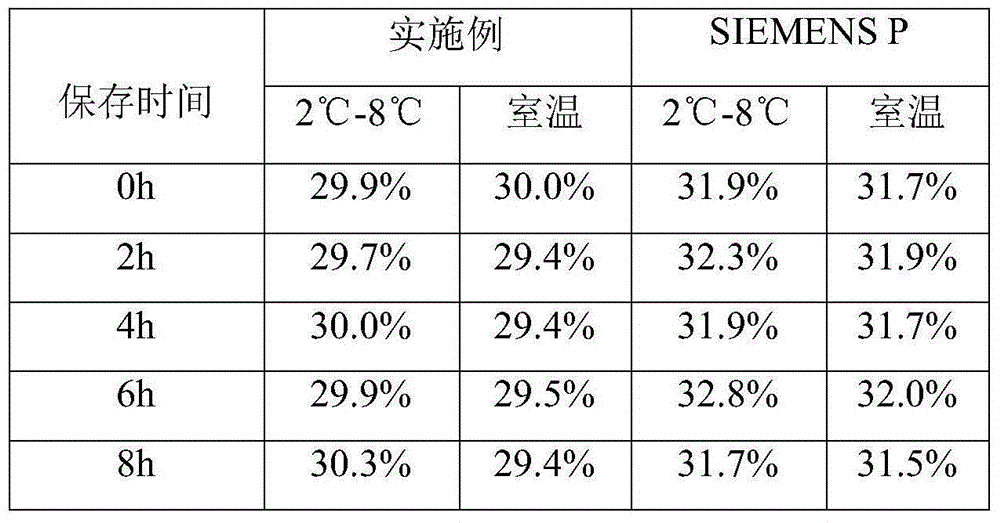
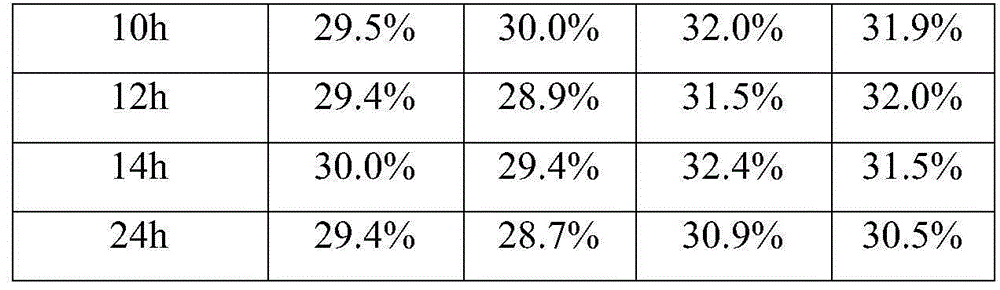
[0016] The embodiment takes the preparation of a blood coagulation factor IX quality control product with an activity of 30% as an example.
[0017] (1) Add and mix the blood collected from human veins and anticoagulant at a volume ratio of 9:1, centrifuge at 2°C to 8°C for 15 minutes, and the relative centrifugal force is 2500g, separate and absorb the upper layer of plasma, and separate the plasma from several people Mixing, that is, multiple servings of mixed plasma; the anticoagulant is trisodium citrate solution, and its concentration is 0.109mol / L to 0.129mol / L.
[0018] (2) The immunoaffinity chromatography column is pre-balanced with a buffer at 10°C to 18°C, and the buffer includes 0.02mol / LTris, 0.15mol / LNaCl, 8mmol / LMgCl 2 , pH7.25.
[0019] (3) Adding MgCl to the mixed plasma of multiple persons 2 Afterwards, the MgCl in multiple pooled plasma 2 The concentration is 4.5-5.5mmol / L....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com