Patents
Literature
46 results about "Protein virus" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Foot-and-mouth disease virus capsid protein tandem coexpressions and virus-like particle preparation method
ActiveCN104404074AHigh activityNatural binding activityBacteriaInactivation/attenuationEscherichia coliVirus-like particle
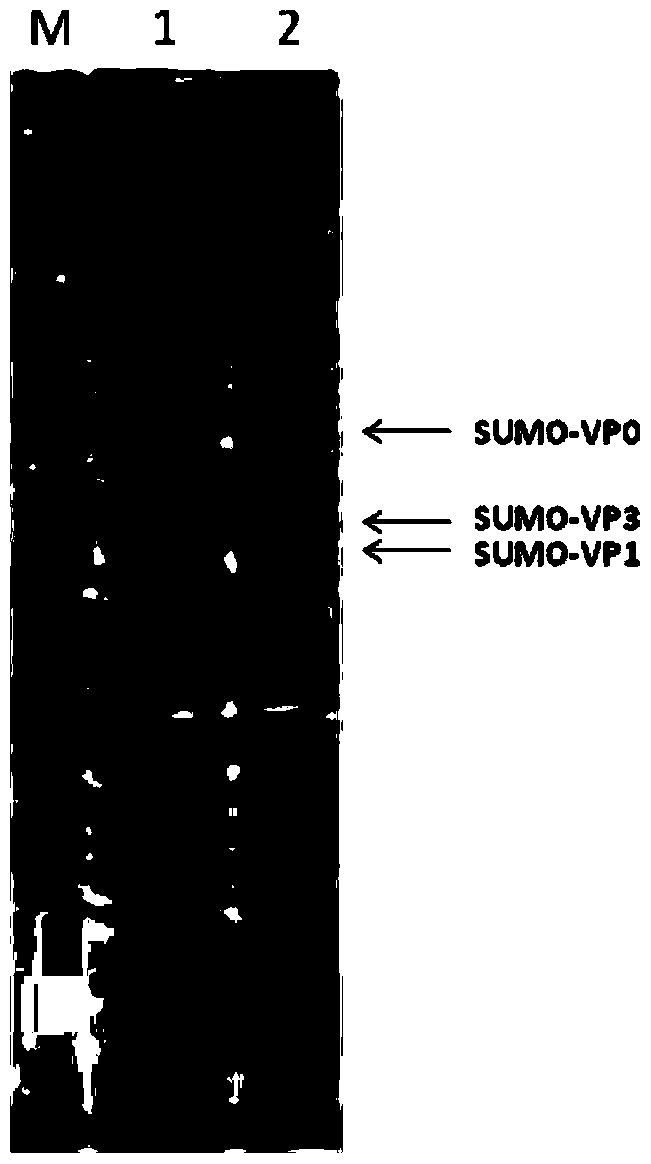
The invention relates to escherichia coli-derived single-plasmid-tandem soluble coexpression foot-and-mouth disease virus capsid proteins VP0 (which is a VP4 and VP2 fusion gene), VP1 and VP3, and a foot-and-mouth disease virus capsid protein virus-like particle preparation method. Foot-and-mouth disease virus capsid protein virus-like particles can be used for preparation of a foot-and-mouth disease vaccine. According to the method, a plurality of aspects of escherichia coli-derived soluble coexpression foot-and-mouth disease virus capsid protein are studied, by comprehensive use of tandem coexpression and SUMO(suggested upper merged ontology) technology with a tag for soluble coexpression of the foot-and-mouth disease virus capsid proteins VP0 (which is the VP4 and VP2 fusion gene), VP1 and VP3, the ultimate objective protein accounts for about 20% of total bacterial protein, and the foot-and-mouth disease virus capsid proteins obtained by purification can be successfully assembled into the virus like particles.
Owner:SA BIOTECH (SUZHOU) PTE LTD
Preparation of PCV2 ORF2 capsid protein virus-like particles derived from escherichia coli
InactiveCN103173470AKeep natural activityHigh activityBacteriaViral antigen ingredientsEscherichia coliOrf2 gene
The invention relates to porcine circovirus PCV2 ORF2 gene optimized by using escherichia coli expression codon and a preparation method of PCV2 ORF2 capsid protein virus-like particles derived from the escherichia coli.
Owner:SA BIOTECH (SUZHOU) PTE LTD
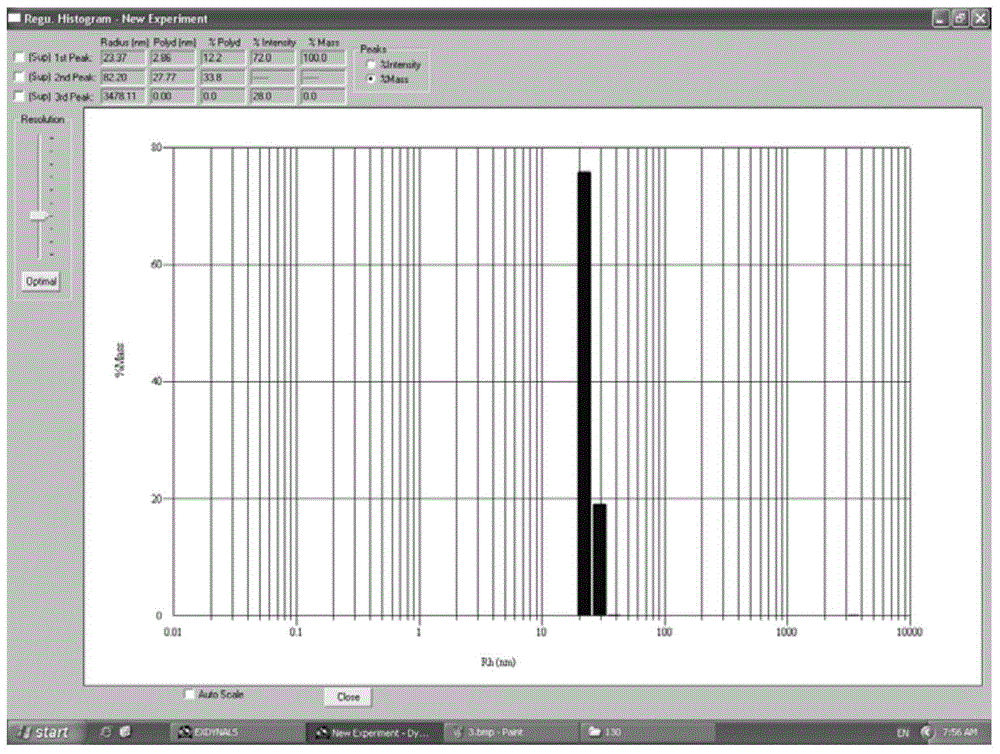
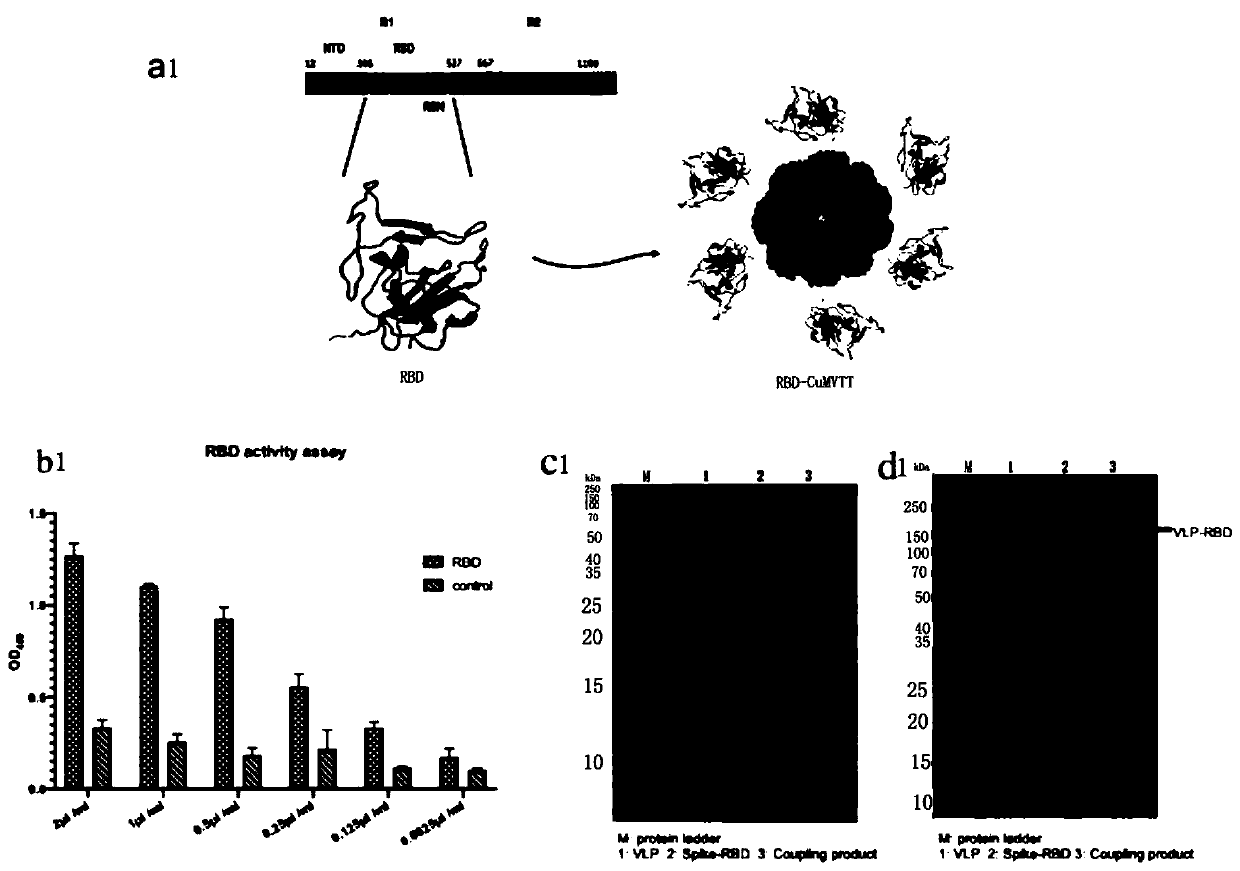
COVID-19-S-RBD virus-like particles and vaccine, and preparation methods of virus-like particles and vaccine
ActiveCN111303255ASuitable for industrial productionEasy to purifySsRNA viruses positive-senseViral antigen ingredientsEscherichia coliImmunity
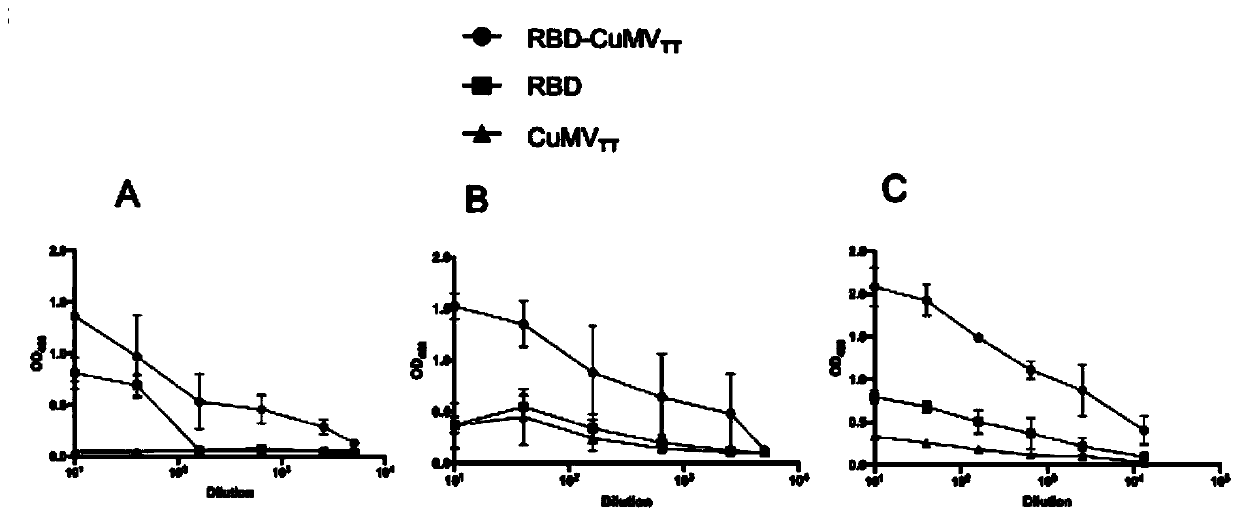
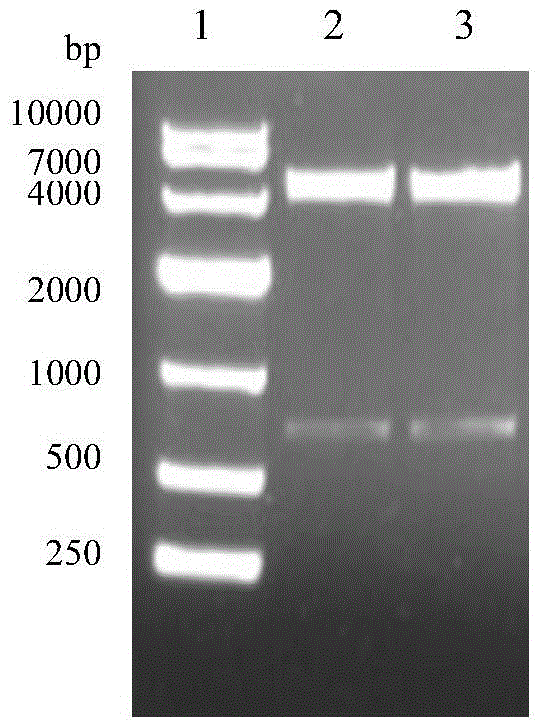
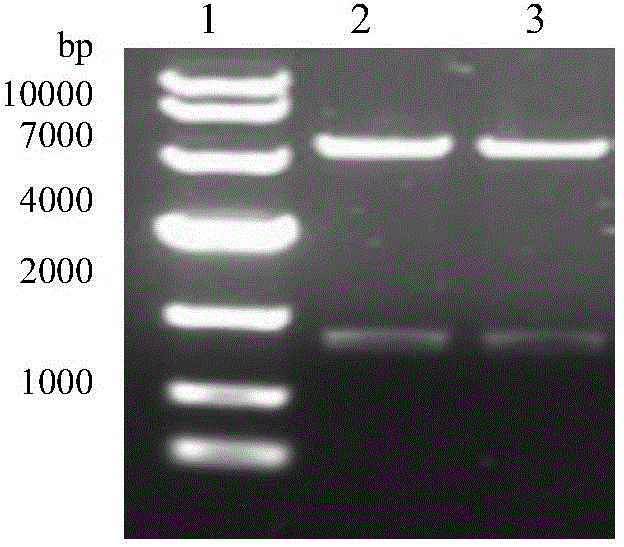
The invention discloses COVID-19-S-RBD virus-like particles and vaccine, and preparation methods of the virus-like particles and the vaccine. pET28a-CuMVTT recombinant plasmid is constructed after a CuMVTT gene is used to connect pET28a plasmid; pFUSE-COVID-19-S-RBD recombinant plasmid is constructed by a COVID-19-S-RBD gene and pFUSE plasmid; the recombinant plasmid is respectively transferred into the expression strains of escherichia coli and the expression cell lines of 293 F cells; the expression strains of escherichia coli are cultivated, biomass is separated through centrifugation, andthe virus-like particles are obtained; COVID-19-S-RBD protein is obtained by cultivating the expression cell lines of the 293 F cells; and the virus-like particles are coupled to the COVID-19-S-RBD through a chemical coupling reagent SMPH. The virus-like particles and the vaccine can be easily obtained through bacteria culture; yield can be higher than that of chimeric expression; and thus, industrial production and fast immunity can be achieved.
Owner:深圳赫兹生命科学技术有限公司
Chimeric protein, virus-like particle and application thereof
ActiveCN104693310AImproving immunogenicityFast and Efficient CarryBacteriaViral antigen ingredientsCircovirusVirus-like particle
The invention provides a chimeric protein, a virus-like particle and application thereof and belongs to the field of molecular biology. The chimeric protein is prepared by the following steps: replacing one, two or three of four sites in a porcine circovirus 2 type Cap protein by a main B cell epitope of a porcine O-shaped foot-and-mouth disease virus VP1 protein to obtain 58th-66th amino acid residues, 72nd-94th amino acid residues, 122nd-147th amino acid residues and 162nd-197th amino acid residues. The chimeric protein can form the chimeric virus-like particle after soluble expression, shows the main B cell epitope of the porcine O-shaped foot-and-mouth disease virus VP1 protein on the surface of the chimeric virus-like particle, has very good immunogenicity to both PCV2 and FMDV and can generate a high antibody to the PCV2 and the FMDV, by once immune.
Owner:江苏省苏农科技术转移中心有限公司
Method for preparing human blood coagulation factors IX and VII subcutaneously from cold-glue-removed blood plasma
InactiveCN105330736AIncrease profitSimple processPeptide preparation methodsBlood coagulation/fibrinolysis factorsUltrafiltrationEngineering
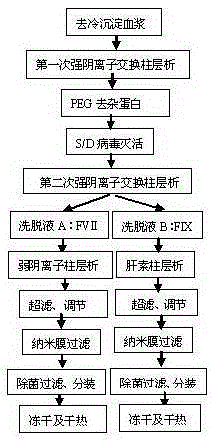
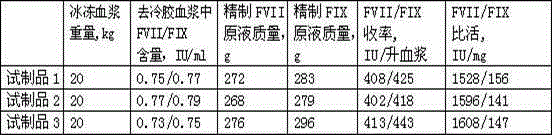
The invention discloses a method for preparing human blood coagulation factors IX and VII subcutaneously from cold-glue-removed blood plasma. The method comprises the following steps: 1, removing cold glue from blood plasma; 2, conducting strong anion-exchange column chromatography the first time; 3, conducting PEG sedimentation for removing impure protein; 4, conducting S / D viral inactivation; 5, conducting strong anion-exchange column chromatography the second time, and obtaining FVII eluent and FIX eluent; 6, conducting weak anion-exchange column chromatography, and concentration for purifying blood coagulation FVII; 7, conducting heparin affinity column chromatography for purifying blood coagulation FIX; 8, conducting ultrafiltration; 9, adding a stabilizing agent, and conducting adjustment; 10, conducting virus-removal filtration through nanofilms; 11, conducting sterilization, filtration and subpackage; 12, conducting freeze-drying; 13, conducting dry-hot viral inactivation. According to the method, PEG sedimentation is adopted for removing the impure protein, the target of preparing high-purity FVII and FIX simultaneously is achieved through combination of an ion-exchange column chromatography technology and an affinity chromatography technology, the process flow is simple, the production cycle is short, a product is subjected to three steps of virus eradicating measures, and use safety is high.
Owner:上海洲跃生物科技有限公司
Preparation method of high-purity human coagulation factor IX
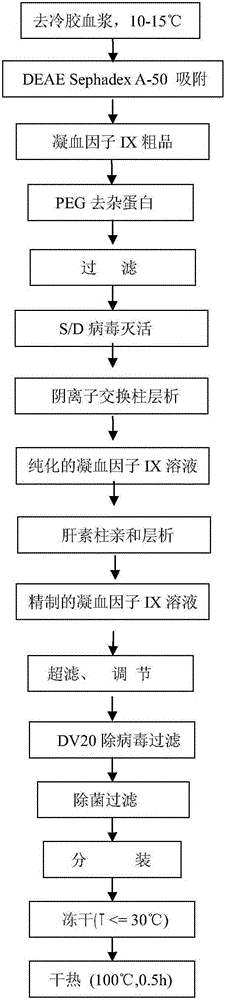
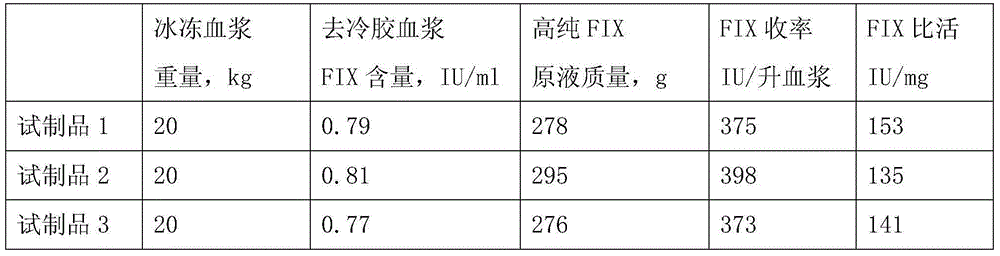
InactiveCN105175486AReduce lossesPrevent denaturation and inactivationPeptide preparation methodsUltrafiltrationPolyethylene glycol
The invention relates to a preparation method of a high-purity human coagulation factor IX, which comprises the following steps: melting refrigerated plasma, and carrying out low-temperature centrifugation; adsorbing with a DEAE (diethylaminoethanol) Sephadex A-50 gel to remove the coagulation factor IX in the cold-glue plasma; removing impure proteins in the solution by using polyethyleneglycol; carrying out S / D virus inactivation; carrying out anion exchange column chromatography to obtain a purified coagulation factor IX solution; passing through a heparin affinity column for further chromatography to obtain a high-purity coagulation factor IX solution; carrying out ultrafiltration, dialysis and concentration, and adding arginine hydrochloride and glycinate as protective agents; filtering through a 20nm filter element to remove viruses; carrying out freeze-drying; and carrying out dry heat virus inactivation. The protein protective agents are added during the gel adsorption, column chromatography and ultrafiltration dialysis, thereby lowering the activation probability of the FIX product thrombin and enhancing the qualification rate of the product. The technique has high product yield; the FIX specific activity can reach 150 IU / mg or so which is much higher than that of the traditional product; and by performing the three-step virus inactivation, the product is safe and reliable to use.
Owner:上海洲跃生物科技有限公司
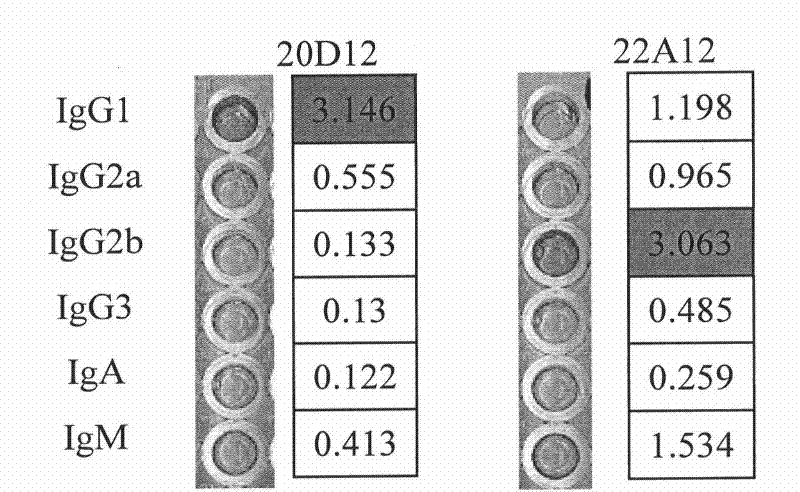
Monoclonal antibody BTV8-VP2-3E11 resistant to bluetongue virus serum 8 type VP2 protein, B-cell epitope peptide identified thereby and application
The invention discloses a monoclonal antibody BTV8-VP2-3E11 resistant to bluetongue virus 8 type (BTV8) VP2 protein, B-cell epitope peptide identified thereby and application, and belongs to the field of BTV8 control. A selected hybridoma cell strain capable of stably secreting a BTV8-VP2 protein resistant monoclonal antibody is stored with a microbial preservation number of CGMCC (China General Microbiological Culture Collection Center) No.7004. The experiment results show that the monoclonal antibody BTV8-VP2-3E11 secreted by the hybridoma cell strain can perform idiosyncratic reaction with the BTV8-VP2 protein but not reacts with the VP2 proteins of other serum types. The monoclonal antibody BTV8-VP2-3E11 and the BTV8-VP2 protein virus specific conserved B cell epitope peptide identified by the a monoclonal antibody can be prepared into an agent used for diagnosing BTV8 infection, thus laying a good foundation for creating serology differential diagnosis methods for BTV8 and other types of serum.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Preparation method of newcastle disease glucoprotein virus antigen and product thereof
ActiveCN104059927ASsRNA viruses negative-senseViral antigen ingredientsImmune protectionRecombinant virus
The invention discloses a preparation method of newcastle disease glucoprotein virus antigen and product thereof. According to the preparation method, the F gene and HN gene of the newcastle disease virus are optimized according to virus prevalent trend prediction, a newcastle disease virus glucoprotein antigen gene and an optimized antigen gene or series-connected optimized antigen genes are combined in a bombyx mori bioreactor for expression, and the expressed antigen or prepared recombinant virus can provide effective immune protection for animals. The invention also provides a preparation method of a newcastle disease virus antigen gene carrying vector. The preparation method comprises the following steps of: cloning the optimized newcastle disease virus antigen gene or series-connected optimized antigen genes into the baculovirus carrying vector controlled by a mammal promotor in a combined way; and recombining to obtain the gene carrying vector controlled by the mammal promotor. After the newcastle disease virus antigen gene carrying vector enters an animal body in an injection or oral administration mode, the newcastle disease virus antigen gene carrying vector can be used for efficiently carrying the antigen gene in the animal body and effectively defending the attack of newcastle disease virus.
Owner:THE INST OF BIOTECHNOLOGY OF THE CHINESE ACAD OF AGRI SCI
Hybridoma cell line and anti-canine distemper virus N protein monoclonal antibody produced through hybridoma cell line
InactiveCN103992988AImmunoglobulins against virusesMicroorganism based processesMicroorganism preservationMicroorganism
The present invention discloses a hybridoma cell line and an anti-canine distemper virus N protein monoclonal antibody CDV-1N8 produced through the hybridoma cell line, and belongs to the field of CDV prevention and treatment. According to the present invention, the hybridoma cell line stably secreting the anti-CDV N protein monoclonal antibody is screened, wherein the microorganism preservation number is CGMCC No.8793, and experiment results prove that the monoclonal antibody CDV-1N8 secreted by the hybridoma cell line can specifically react with the CDV N protein, and does not react with the Vero cell protein; the monoclonal antibody can specifically recognize the CDV-N protein, and accurate positioning of the CDV-N protein B-cell epitope recognized by the monoclonal antibody is QITFLHSERS; and the monoclonal antibody CDV-1N8 and the CDV N protein virus-specific conservative B cell epitope polypeptide recognized by the monoclonal antibody can be made into the CDV infection diagnosis reagent so as to establish the foundation for establishment of the CDV serological differential diagnosis method.
Owner:JL TEYAN BIOLOGICAL TECH LIMITED LIABILITY +1
Method of preparing porcine parvovirus virus-like particle subunit vaccine by using Escherichia coli expression system and application of method
InactiveCN106148358AImprove solubilityGood specific immune responseBacteriaViral antigen ingredientsEscherichia coliEukaryotic plasmids
The invention discloses an encoding gene of porcine parvovirus VP2 protein, a method of prokaryotically expressing VP2 protein virus-like particles, and application of the method in vaccine preparation. Sequences are optimized, VP2 gene is artificially synthesized, the synthesized gene is inserted into pET28a vector, the gene and chaperone protein plasmids are co-transferred to BL21(DE3) host bacteria, the VP2 protein and chaperone protein are co-expressed to promote correct folding of the VP2 protein. Experiments prove that recombinant bacteria expressed VP2 protein can be self-assembled in vitro and has good immunogenicity; by immunizing mice and guinea pigs with the virus-like particle subunit vaccine prepared with the VP2 protein expressed herein, it is possible to induce the production of a high level of hemagglutination inhibition antibodies and neutralizing antibodies, and the vaccine can prevent guinea pigs from being affected by strong porcine parvovirus. The recombinant bacteria according to the invention can be utilized to efficiently prepare porcine parvovirus virus-like particles, the production cost is low, operation is simple, and biosafety is better.
Owner:HENAN ACAD OF AGRI SCI +1
Vectors and methods for immunization against norovirus using transgenic plants
The present invention relates to a synthetic plant-optimized nucleic acid molecule having a Norwalk virus capsid protein coding nucleotide sequence, and nucleic acid constructs, host cells, expression systems, and plants having the plant-optimized Norwalk virus nucleic acid molecule. The present invention also relates to a method of producing Norwalk virus capsid protein virus-like particles in a transgenic plant or transgenic plant seed transformed with a plant-optimized nucleic acid molecule encoding Norwalk virus capsid protein. The plant or a component thereof can be administered to a subject under conditions effective to immunize the subject against disease resulting from infection by a Norovirus, including Norwalk virus. An oral vaccine for immunization of a subject against Norwalk virus infection is also disclosed.
Owner:BOYCE THOMPSON INST FOR PLANT RES
Method and composition for the diagnosis of equine infectious anemia virus disease by using the recombinant capsid protein virus (p26)
InactiveUS6596846B2Microbiological testing/measurementPeptide preparation methodsSerum igeEquine infectious anemia
The present invention relates to a method and kit for detecting antibodies in clinical samples of animals infected with equine infectious anemia virus using the immunodiagnosis with the recombinant viral antigen p26. The antigen was bound to solid supports (microtitter plates, tubes, beads or nitrocellulose papers or nylon) and reacted with the test serum. After incubation with conjugated anti-equine immunoglobulin-enzyme the reaction was revealed with a solution composed of the substrate of the enzyme used in the conjugate (cromogene). After development of the reaction (color formation) it was stopped with acid solution and measured. The immunoassay may be a direct second antibody immunoassay, a one or two step sandwich immunoassay.
Owner:UNIVERSIDADE FEDERAL DE MINAS GERAIS
Tandem co-expression of foot-and-mouth disease virus capsid protein and preparation method of virus-like particles
Owner:SA BIOTECH (SUZHOU) PTE LTD
Vaccine composition and preparation method thereof
ActiveCN104623653AImprove efficiencyViral antigen ingredientsAntiviralsVirus-like particleProtein virus
The invention provides a vaccine composition, which comprises an immunizing dose of PCV2Cap protein virus-like particle antigen and a stable buffer solution. In the vaccine composition, the PCV2Cap protein can stably exist in the form of virus-like particles, and after preservation of different time, the effect is obviously better than a contrast vaccine.
Owner:PU LIKE BIO ENG +1
Monoclonal antibody BTV8-VP2-4D9 resist ant to bluetongue virus serum 8 type VP2 protein, B-cell epitope peptide identified thereby and application
The invention discloses a monoclonal antibody BTV8-VP2-4D9 resistant to bluetongue virus 8 type (BTV8) VP2 protein, B-cell epitope peptide identified thereby and application, and belongs to the field of BTV8 control. A selected hybridoma cell strain capable of stably secreting a BTV8-VP2 protein resistant monoclonal antibody is stored with a microbial preservation number of CGMCC (China General Microbiological Culture Collection Center) No.7003. The experiment results show that the monoclonal antibody BTV8-VP2-4D9 secreted by the hybridoma cell strain can perform idiosyncratic reaction with the BTV8-VP2 protein but not reacts with the VP2 proteins of other serum types. The monoclonal antibody BTV8-VP2-4D9 and the BTV8-VP2 protein virus specific conserved B cell epitope peptide identified by the a monoclonal antibody can be prepared into an agent used for diagnosing BTV8 infection, thus laying a good foundation for creating serology differential diagnosis methods for BTV8 and other types of serum.
Owner:HARBIN VETERINARY RES INST CHINESE ACADEMY OF AGRI SCI
Expression and purification of murine norovirus VP1 protein virus-like particles and preparation of polyclonal antibody
InactiveCN107988240AFully formedStable formationSsRNA viruses positive-senseSerum immunoglobulinsLaboratory mouseNew Zealand white rabbit
The invention discloses expression and purification of murine norovirus VP1 protein virus-like particles and preparation of a polyclonal antibody. According to the technical scheme, structural proteinVP1 genes of the murine norovirus are subjected to codon optimization for the first time, protein is expressed by utilizing a baculovirus expression system, the expressed protein forms the virus-likeparticles, and the antibody can simulate capsid protein of live viruses. For a VLPs antigen, the new Zealand white rabbit is immunized, the polyclonal antibody capable of resisting op.VP1 is prepared, the experiment proves that the polyclonal antibody can be used for Western Blot detection and IFA analysis, and a basic material is provided for detecting the MNV carrying condition of the laboratory mouse in a laboratory. Meanwhile, the VLPs antigen and the polyclonal antibody lay a foundation for further developing an ELISA detection kit.
Owner:NANJING GENERAL HOSPITAL NANJING MILLITARY COMMAND P L A
Replication-defective A virus expression vector system and vaccine preparation method
InactiveCN102443603AReduce the risk of useAvoid not being able to expand cultureViral antigen ingredientsAntiviralsNegative strandViral Vaccine
The invention discloses a replication-defective A virus expression vector system and a vaccine preparation method. The vector system consists of a replication-defective A virus vector of a deleted structural gene and minus strand complementary plasmids or cells containing a controllable virus A structural gene. In the preparation method, an exogenous gene target is introduced by constructing the replication-defective A virus vector of a deleted virus structural protein; and the replication-recombinant A virus can be continuously produced in a large scale by combining the minus strand complementary plasmids or cells containing the controllable A virus structural gene, so that the problem of low virus yield of a conventional A virus system can be solved, a target of preparing recombinant virus in a large scale is realized, and exogenous protein and virus sample particles can be expressed in scale. The replication-defective A virus expression vector system disclosed by the invention is suitable for large-scale production of DNA (deoxyribonucleic acid) vaccine, vector virus vaccine and exogenous protein expression.
Owner:SOUTH CHINA UNITED VACCINE INST
Method for transfecting cells through ultrasonic perforation
ActiveCN112266930AEasy to operateHigh transfection efficiencyNucleic acid vectorElectrical/wave energy microorganism treatmentUltrasound - actionProtein target
The invention discloses a method for transfecting cells through ultrasonic perforation. The method comprises the following steps: firstly, culturing target cells; secondly, mixing the target cells, atarget object and an ultrasonic contrast agent in a container; thirdly, applying ultrasonic waves to the container; and fourthly, re-culturing the container under a constant temperature condition, sothat the target object fully enters the target cells, wherein the target object is a target gene or a target protein, and when the target object is the target gene, the frequency of the ultrasonic waves adopted in the third step is 800-900 kHz; and when the target object is the target protein, the frequency of the ultrasonic waves adopted in the third step is 800-1200 kHz. The method for transfecting cells through ultrasonic perforation is simple to operate, high in transfection efficiency and low in cost, and can be used for converting or transfecting molecules such as DNA (Deoxyribonucleic Acid), RNA (Ribose Nucleic Acid), proteins, viruses, sugar, medicines and nanoparticles in an unlimited range.
Owner:SUZHOU INST OF BIOMEDICAL ENG & TECH CHINESE ACADEMY OF SCI
Monoclonal antibody for hand-foot-mouth EV71 virus and application thereof
ActiveCN101812129BStrong specificityIncreased sensitivityImmunoglobulins against virusesTissue cultureProtein targetCarrier protein
The invention provides a monoclonal antibody for hand-foot-mouth EV71 virus, which is obtained through immunogen preparation by using VP1 protein of the EV71 virus as target protein to design and synthesize a polypeptide sequence and coupling the polypeptide sequence with vector protein serving as immunogen. The monoclonal antibody has no cross reaction with other proteins of the EV71 virus, CA16virus, other antigens or pathogens, has the advantages of high specificity and high sensitivity in detection, can accurately detect the level of the EV71 virus in a detected sample, and is expected to be widely applied in clinical detection.
Owner:ABMAX BIOTECHNOLOGY CO LTD
Method for treating infectious diseases using emissive energy
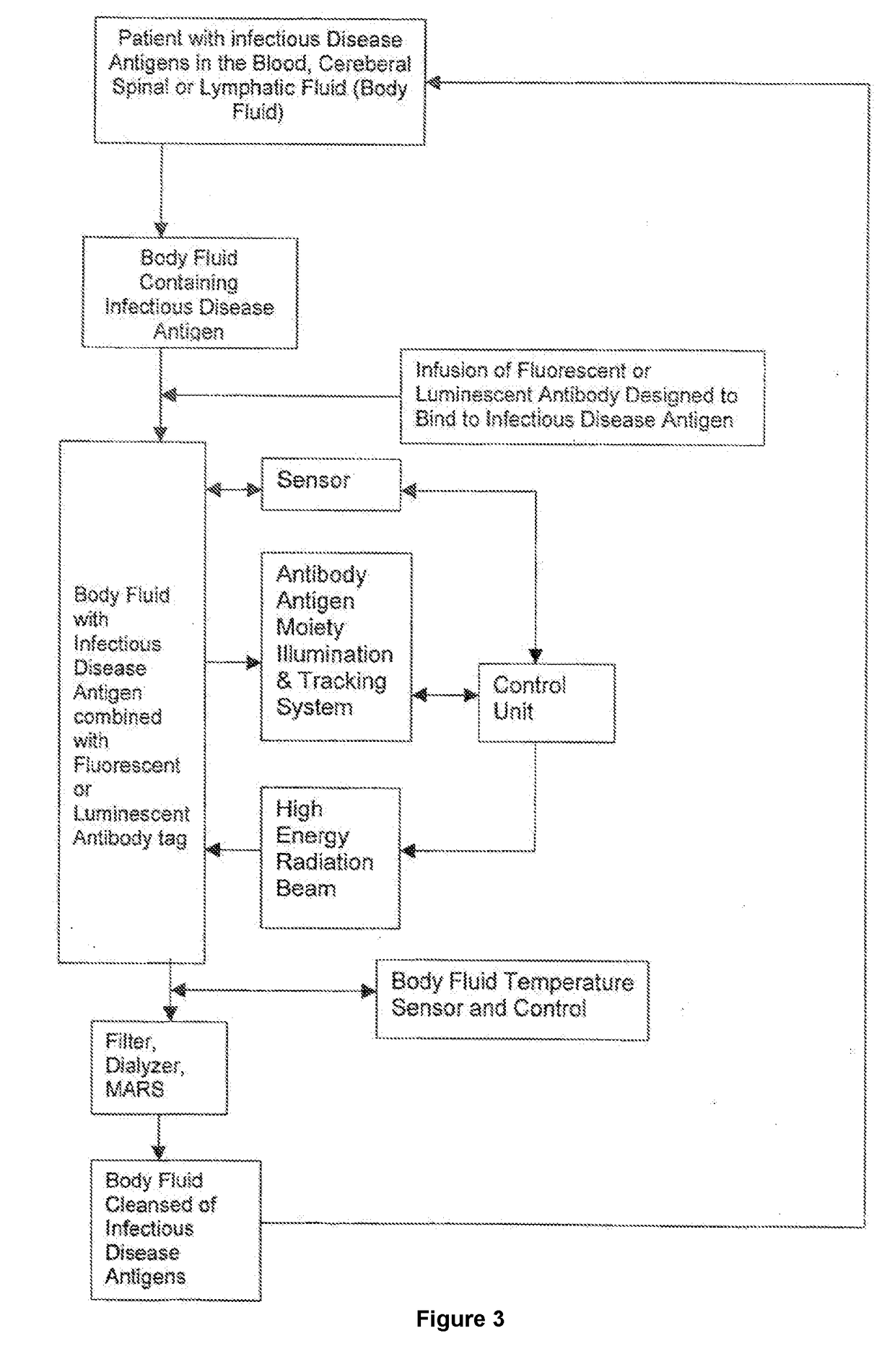
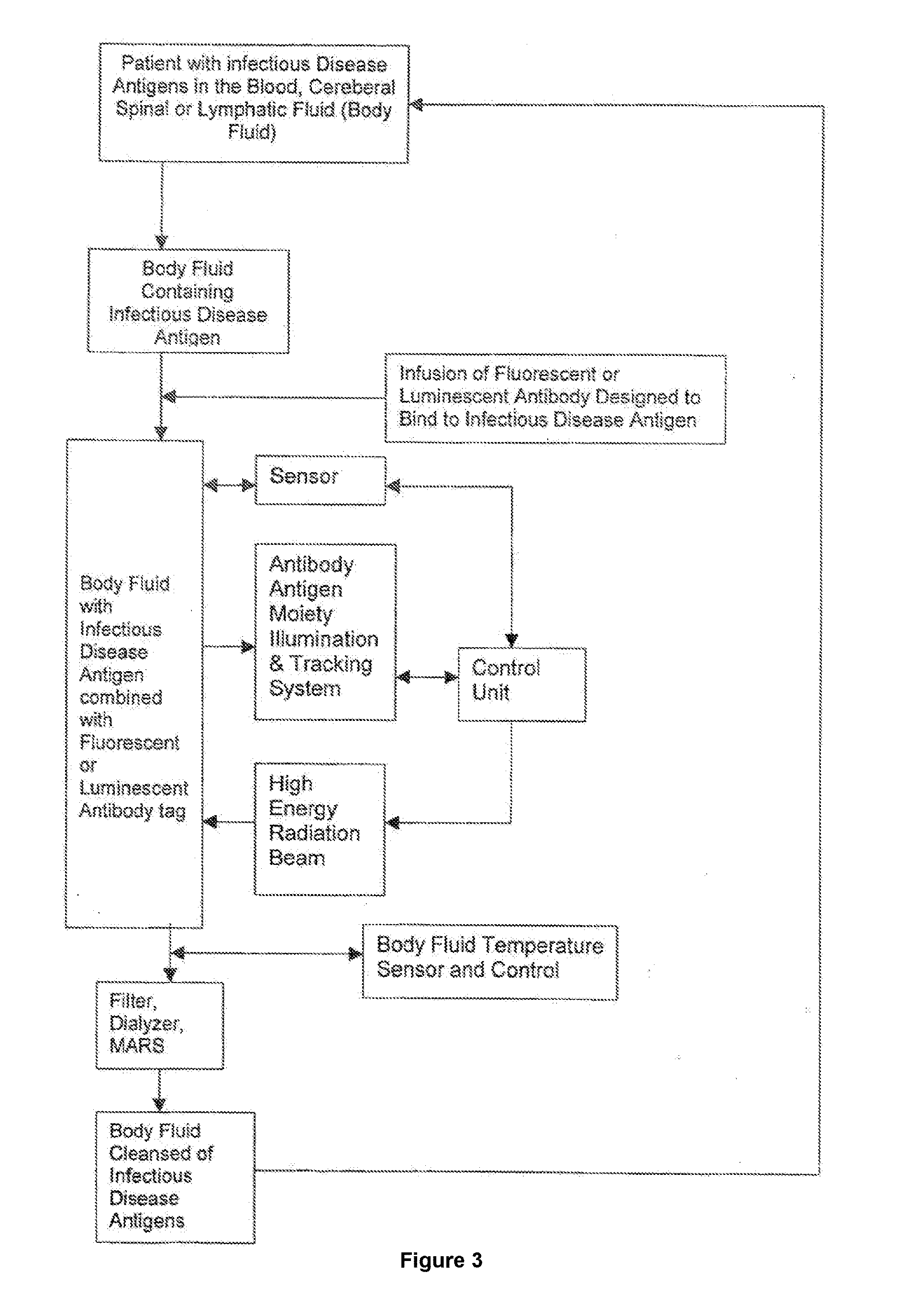
InactiveUS20180078641A1Energy modified materialsOther blood circulation devicesProtein targetCancer cell
The present invention relates to the treatment of infectious diseases, specifically by extracorporeally eradicating the pathogen. This invention comprises methods for the extracorporeal treatment of infectious diseases that will remove infectious pathogens (leukemia cells, bacteria, viruses, or fungi causing a septicemia, metastatic cancer cells, target protein, viruses, parasites, fungi and prions) in humans by targeting such pathogens with a laser or other high-energy source of emissive radiation. More specifically, the method involves removing a bodily fluid from a patient, attaching an antibody to pathogens in the bodily fluid, sensing the antibody-pathogen moiety, using a high-powered, focused laser, or other suitable light source, to destroy the antibody-pathogen moiety, removing the remains of the antibody-pathogen by filtering or other suitable mechanism(s), and returning the bodily fluid to the patient.
Owner:MARV ENTERPRISES
IBV-III antigen production process
The invention supplies a manufacturing technique of IBV-HI antigen, the preparation process of antigen is that: the virus fluid of containing the protein virus is isolated from chicken embryo allantois fluid of containing respiratory type IBV the virus and blending with clostridium filtrate of rabbits A type wei surname as a culture medium, after two hours water bath action with 37deg.C, put the fluid into the 4deg.C fridge for 48 hours, through freeze drying to get antigen, the related centrifuge divided into two centrifugal, first the mixed fluid through high speed centrifugation, choose the supernatant after the centrifugation; then it progress the two centrifugation as ultracentrifugation, the time is 2-3h, the rotation rate of centrifugation is 25000-35000g, abandon the supernatant after the centrifugation, and use deposit suspension to obtain virus fluid. Using this invention method, infectious bronchitis HI antigen of respiratory system has relatively low cost, type specificity, high sensitivity, stability feature, it makes HI method to detect respiratory infectious bronchitis feasible, and enhance the credibility and maneuverability of its detection.
Owner:秦卓明 +2
Preparation method of porcine circovirus type 3 (PCV-3) Cap protein virus-like particles and application thereof
InactiveCN110684084APromote formationImprove expression efficiencyViral antigen ingredientsVirus peptidesSpecific immunityPorcine Circoviruses
The invention discloses a preparation method of porcine circovirus type 3 (PCV3) Cap protein virus-like particles and an application thereof. The method is based on comparative analysis of Cap proteingene sequences of PCV3 epidemic strains, a yeast expression signal peptide sequence is added to the front end of an artificially synthesized PCV3-Cap protein coding gene, codons of the Cap gene are optimized, a recombinant saccharomyces cerevisiae expression strain is constructed by using a saccharomyces cerevisiae transcription element efficient construction method, and virus-like particles of the recombinant porcine circovirus are generated through induced expression. A subunit vaccine is prepared from the Cap protein expressed by the recombinant saccharomyces cerevisiae expression strain,an organism can be induced to generate specific immune response after animals are immunized, and a pig body can be protected against attack of the porcine circovirus.
Owner:TIANJIN RINGPU BIO TECH
Method for treating infectious diseases using emissive energy
InactiveUS20170049889A1Other blood circulation devicesEnergy modified materialsCancer cellProtein target
The present invention relates to the treatment of infectious diseases, specifically by extracorporeally eradicating the pathogen. This invention comprises methods for the extracorporeal treatment of infectious diseases that will remove infectious pathogens (leukemia cells, bacteria, viruses, or fungi causing a septicemia, metastatic cancer cells, target protein, viruses, parasites, fungi and prions) in humans by targeting such pathogens with a laser or other high-energy source of emissive radiation. More specifically, the method involves removing a bodily fluid from a patient, attaching an antibody to pathogens in the bodily fluid, sensing the antibody-pathogen moiety, using a high-powered, focused laser, or other suitable light source, to destroy the antibody-pathogen moiety, removing the remains of the antibody-pathogen by filtering or other suitable mechanism(s), and returning the bodily fluid to the patient.
Owner:MARV ENTERPRISES
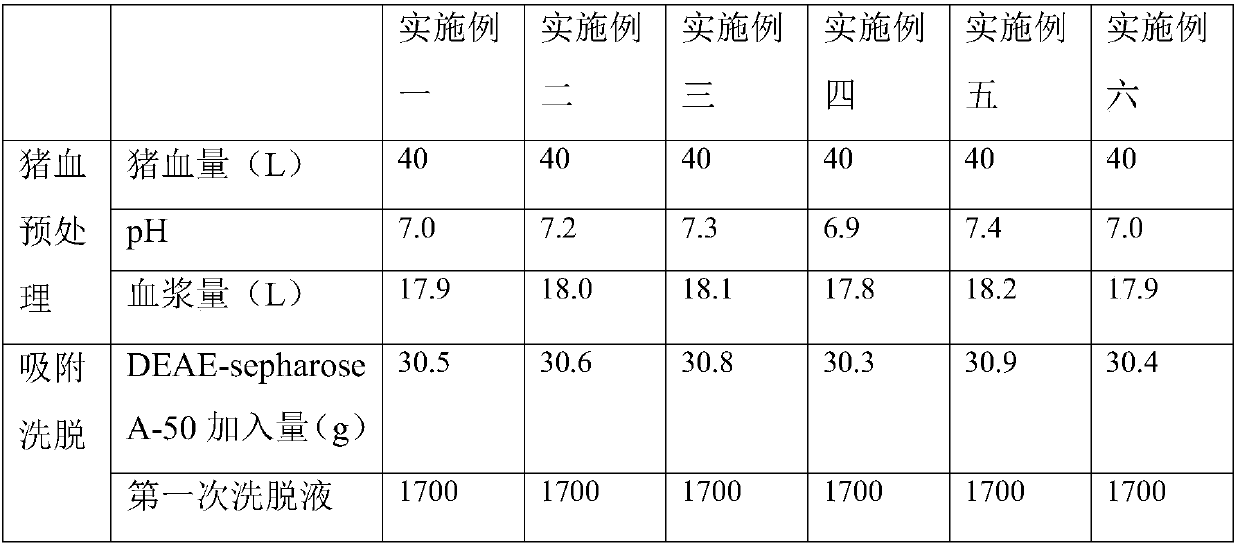
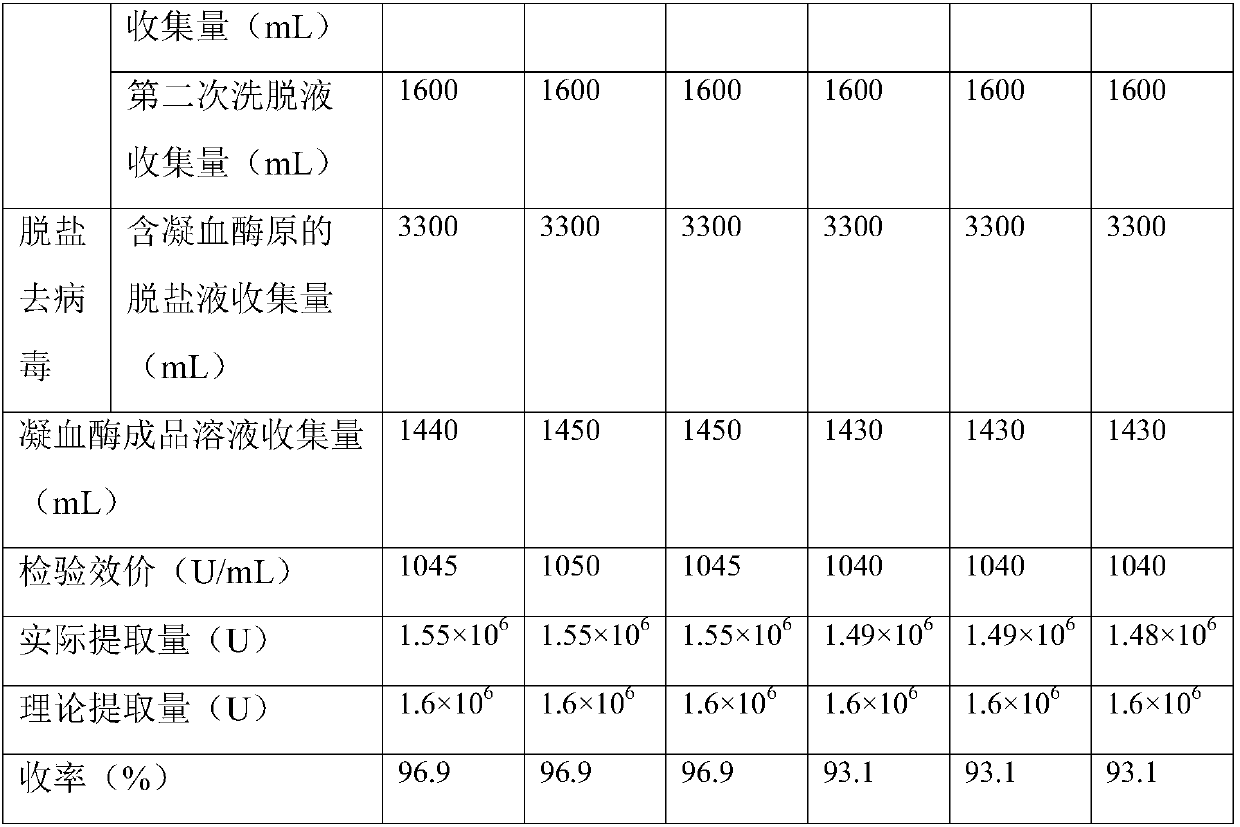
Preparation method for porcine thrombin
The invention discloses a preparation method for porcine thrombin. The invention aims to solve the problems of high operation difficulty and being adverse to industrial production of the thrombin extraction. The key point of the technical scheme comprises the following steps: adding trisodium citrate liquid into pig blood, thereby acquiring a pig blood-trisodium citrate mixed liquid; centrifugallylayering the pig blood-trisodium citrate mixed liquid, thereby acquiring the plasma of pig blood; adding anion exchanger into the plasma of the pig blood, stirring and absorbing, cleaning the anion exchanger and then filling into an eluting column; preparing a NaCl-trisodium citrate mixed liquid as an eluant, eluting and collecting the eluant containing prothrombin; desalting the eluant, removingsolvent and monitoring the conductivity of the eluant eluted from a desalting column, thereby acquiring a desalted liquid containing prothrombin; adding CaCl2 liquid into an activated liquid containing thrombin; removing viruses, thereby acquiring a thrombin liquid. The impurities, such as, impure proteins and viruses in the thrombin can be effectively reduced and the thrombin purity can be increased.
Owner:ZHEJIANG FENGAN BIOPHARM
Porcine circovirus type 2 (PCV2) Cap protein virus-like particle (VLP) preservation solution and application
A porcine circovirus type 2 (PCV2) Cap protein virus-like particle (VLP) preservation solution is based on a salt ion buffer solution system, saccharides, a nonionic surfactant, a metal ion chelatingagent, alcohols, a protein stabilizer, amino acids and a preservative are added, the PCV2 Cap protein VLP preservation solution can prolong the preservation period of the PCV2 Cap protein effectively,preserve the PVC2 Cap protein for 12 months at 2-8 DEG C while keeping protein concentration and activity unchanged, preserve the PCV2 Cap protein for 36 months at subzero 20 DEG C while keeping protein concentration and activity unchanged, can be used as a preservation solution of a standard protein of a kit for detecting the PCV antigen concentration or a diluted solution of a PCV2 Cap proteinsubunit vaccine.
Owner:BEIJING KEMUFENG BIOLOGICAL PHARMA +3
IBV-III antigen production process
The invention supplies a manufacturing technique of IBV-HI antigen, the preparation process of antigen is that: the virus fluid of containing the protein virus is isolated from chicken embryo allantois fluid of containing respiratory type IBV the virus and blending with clostridium filtrate of rabbits A type wei surname as a culture medium, after two hours water bath action with 37deg.C, put the fluid into the 4deg.C fridge for 48 hours, through freeze drying to get antigen, the related centrifuge divided into two centrifugal, first the mixed fluid through high speed centrifugation, choose the supernatant after the centrifugation; then it progress the two centrifugation as ultracentrifugation, the time is 2-3h, the rotation rate of centrifugation is 25000-35000g, abandon the supernatant after the centrifugation, and use deposit suspension to obtain virus fluid. Using this invention method, infectious bronchitis HI antigen of respiratory system has relatively low cost, type specificity, high sensitivity, stability feature, it makes HI method to detect respiratory infectious bronchitis feasible, and enhance the credibility and maneuverability of its detection.
Owner:秦卓明 +2
Canine parvovirus trivalence subunit vaccine
PendingCN110157687ASolve the problem of not being able to provide comprehensive protectionHigh antibody titerViral antigen ingredientsAntiviralsAntigenNucleotide
The invention provides a canine parvovirus trivalence subunit vaccine. Antigens are VP2 protein virus-like particles of a canine parvovirus and are obtained by conducting cultivation and collection after a recombinant rhabdovirus is utilized for infecting insect cells; the recombinant rhabdovirus carries nucleotide fragments for coding VP2 proteins; the amino acid sequences of the VP2 proteins areSEQ ID NO:7, SEQ ID NO:8 and SEQ ID NO:9 respectively. After the prepared vaccine is utilized for immunizing a canine, the antibody titer of the canine can be increased, and infection of the canine parvovirus is prevented.
Owner:YEBIO BIOENG OF QINGDAO
Purification of proteins and viral inactivation
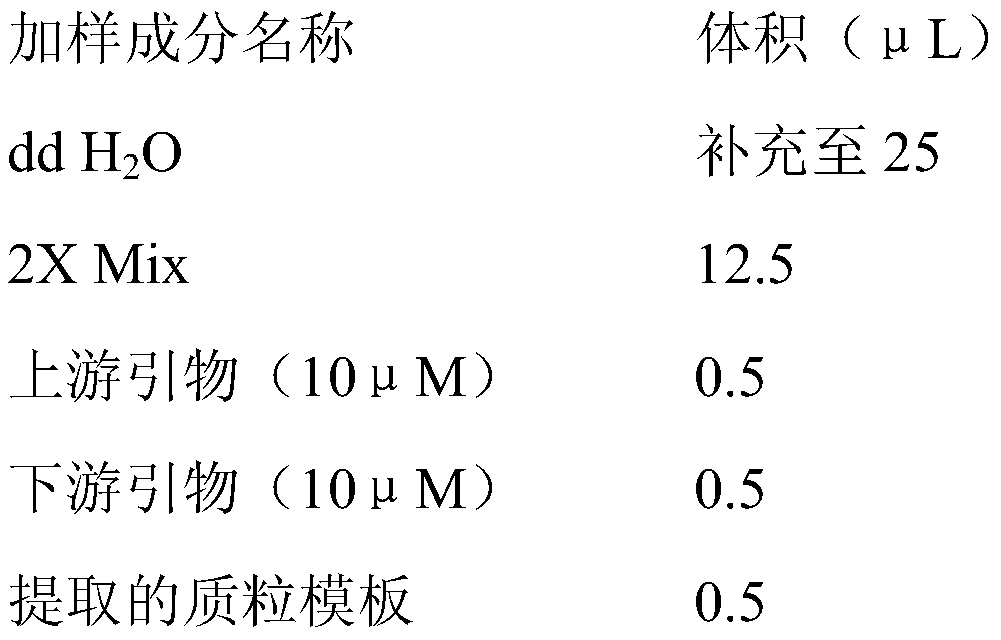
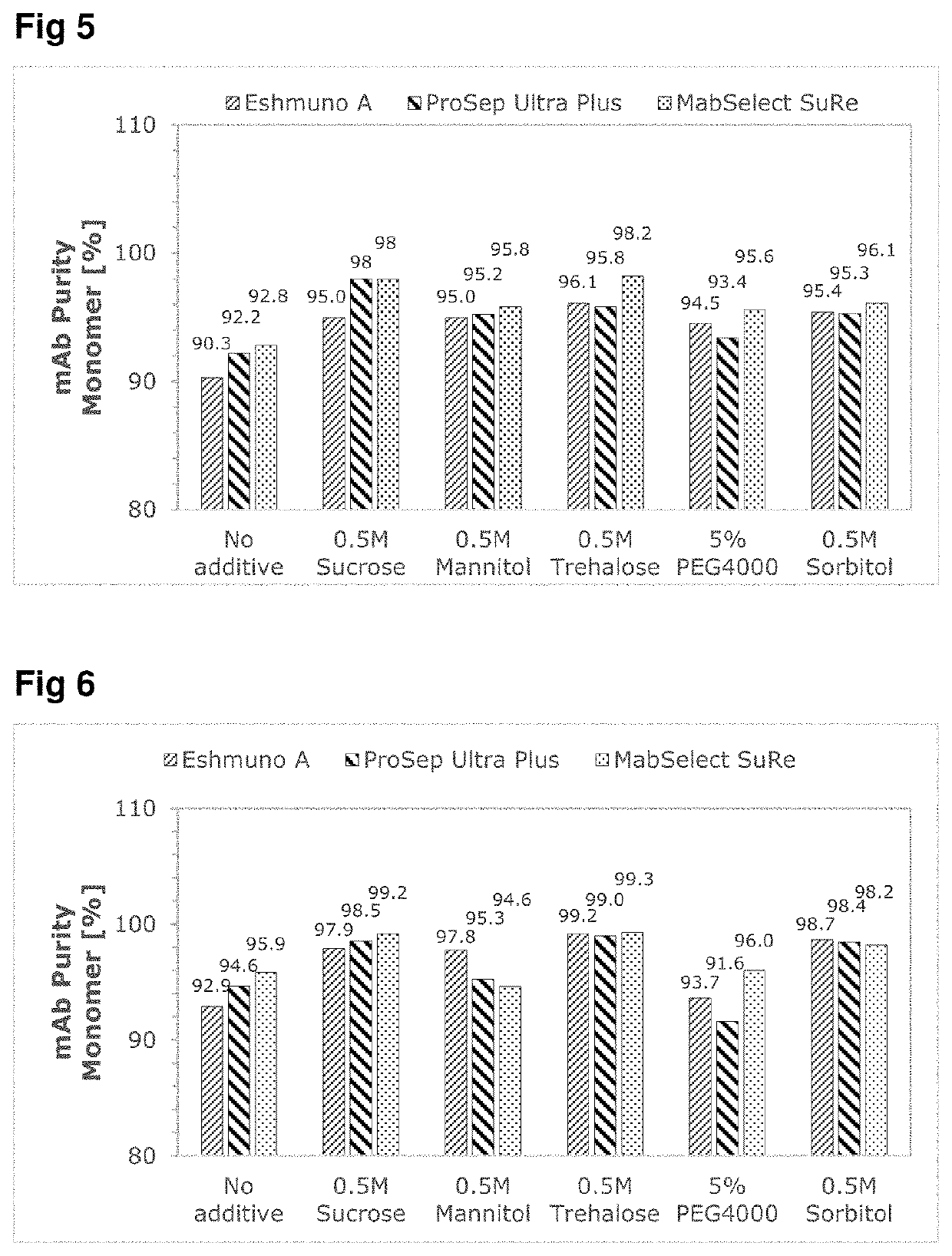
PendingUS20220348608A1Improve production yieldEffectively stabilize mAbsPeptide preparation methodsImmunoglobulinsProtein targetPolythylene glycol
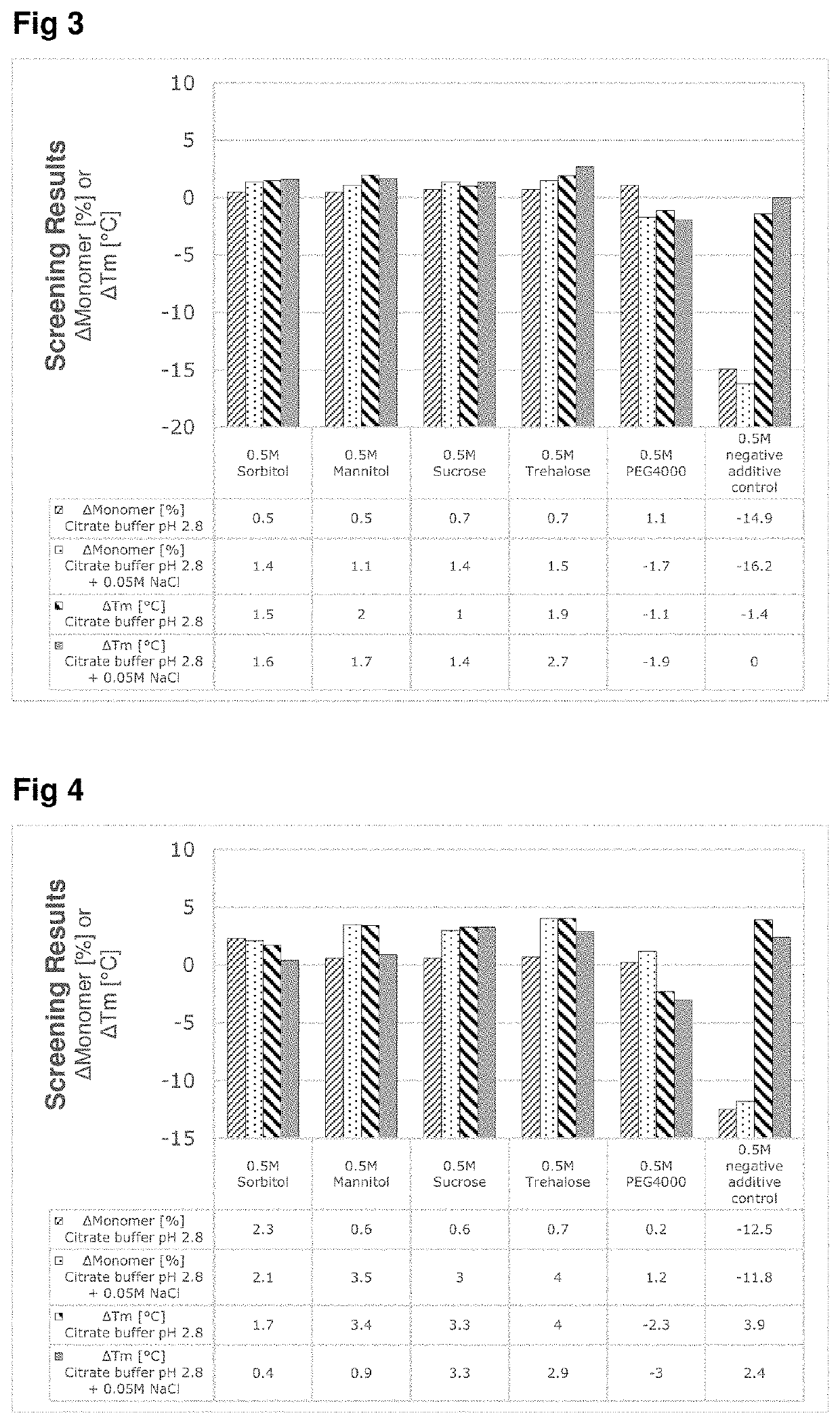
A method for purifying a target protein from a cell culture sample containing the target protein, viral compounds and other impurities, by affinity chromatography virus inactivation and optionally other purifications the affinity chromatography involvinga) loading an affinity chromatography column with the cell culture sample thereby binding the target protein to the affinity chromatography column;b) eluting the target protein from the affinity chromatography column by contacting the affinity chromatography column with an elution buffer having a pH<6 and comprising an excipient, wherein the excipient is disaccharides, polyols or poly (ethylene glycol) polymers;c) collecting one or more fractions containing the target protein obtained from (b);d) potentially combining the fractions obtained from (c) to form an elution product pool,and wherein the virus inactivation involvese) incubating the elution product pool at a pH from 2.5 to 4.5.
Owner:MERCK PATENT GMBH
Recombinant vector, recombinant protein and virus-like particle of human papilloma virus 16-type epitope chimeric L1 as well as preparation and application thereof
InactiveCN111944834AUniform particle sizeFull shapeAntibody mimetics/scaffoldsViral antigen ingredientsEscherichia coliCell immune response
A virus-like particle system containing a human papilloma virus 16 type epitope chimeric DNA fragment is constructed; firstly, an epitope chimeric main capsid protein L1 DNA fragment containing humanpapilloma virus 16 type E749-57 is connected to a plasmid pET28a to construct a recombinant plasmid pET28a-16L1-E749-57, and then is transferred into escherichia coli for culture to obtain recombinantprotein; and self-assembling is carried out, such that virus-like particles (VLPs) are obtained. The assembled epitope chimeric virus-like particles can stimulate humoral and cellular immune responses at the same time.
Owner:JINLIN MEDICAL COLLEGE
Medical nerve grafting repair film and preparation method thereof
The invention discloses a medical nerve grafting repair film and a preparation method thereof. The preparation method comprises the steps of material selection, impure protein removal, virus inactivation, collagen degreasing, casing extension, chitosan atomization, dressing shaping, dressing solidification, cutting and inspection, packaging, sterilization and the like. Chitosan has antibacterial activity, can resist microbial infection, induce tissue regeneration and accelerate growth and healing of nerve fibers, and does not cause any inflammatory reaction in tissue; collagen has hemostatic and repairing functions, has bioabsorbability and cell adhesion, and can promote the formation of new cells and nerve cells; the product is low in antigenicity, good in human tissue compatibility, stable in absorption period through automatic coordination and absorption of enzymes and body fluid, and finally completely absorbed by a human body; the burn and scald repair film prepared by the methodhas the characteristics of uniform texture, good elasticity, high transparency, good air permeability, good biocompatibility, small toxic and side effects and the like, and the dressing can be absorbed by organisms.
Owner:山东康利达医用制品有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com