Vaccine composition and preparation method thereof
A vaccine composition and buffer technology, applied in the field of vaccine compositions, can solve problems such as virus-like particles of vaccine products that are not disclosed, and achieve the effects of removing viruses and improving efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Preparation and inspection of embodiment 1PCV2 subunit vaccine
[0034] 1. Preparation of Cap protein stabilization buffer
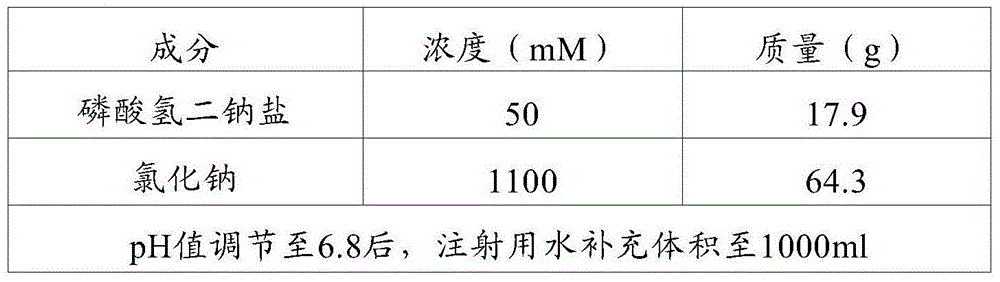
[0035] In this embodiment, the most preferred formula is used to prepare the buffer solution. The specific steps are as follows. Use water for injection to prepare a disodium hydrogen phosphate solution with a concentration of 50 mM, adjust the pH to 6.8 with phosphoric acid, add sodium chloride to make the final concentration 1100 mM, and use it after the solution is completely prepared. Filter through a 0.22 μm filter membrane, seal and store at room temperature for later use. The detailed formulation is shown in Table 1 below.
[0036] Table 1 Preparation of VLPs stabilization buffer (1000ml)
[0037]
[0038] 2. Vaccine preparation
[0039] Add a weighed amount of GEL adjuvant to a sterilized beaker. According to Examples 1-3 of patent CN103173470A, the PCV2 protein virus-like particle antigen was prepared with a content of 1.3 mg / ml. ...
Embodiment 2
[0050] Example 2 PCV2 subunit vaccine prevents and treats the efficacy of PCV2 infection
[0051] 1. Comparative study on the effect of immunizing piglets with different doses of PCV2 subunit vaccine
[0052] In this example, the effects of five PCV2 candidate vaccines were tested, and the efficiency parameters after exposure to virulent PCV2 strains were further determined. Thirty-five 9-14-day-old piglets that did not eat colostrum were randomly divided into 7 groups of the same size, with 5 pigs in each group. Group 1 was injected with 1ml of PCV2 subunit vaccine V1 (60μg / ml) in neck muscle, group 2 was injected with 1ml of PCV2 subunit vaccine V2 (30μg / ml) in neck muscle, and group 3 was injected with PCV2 subunit vaccine V3 in neck muscle (10μg / ml) 1ml, the 4th group intramuscular injection of PCV2 subunit vaccine V4 (5μg / ml) 1ml, the fifth group intramuscular injection of PCV2 subunit vaccine V5 (2μg / ml) 1ml, the sixth group no immunization , as the challenge control...
Embodiment 3
[0119] Embodiment 3 PCV2 subunit vaccine stability research
[0120] Dr. Yin Shuanghui of Lanzhou Veterinary Research Institute, Chinese Academy of Agricultural Sciences reported "Preparation and Immunogenicity of Porcine Circovirus Type 2 Capsid Protein Virus-Like Particles", which used the Escherichia coli expression system to prepare PCV2Cap VLPs as antigen inoculation In pigs, the antibody can turn positive 7 days after immunization, and the antibody still shows an upward trend until 28 days after immunization. According to its technical scheme and route, we constructed His-SUMO-Cap, and obtained natural Cap protein through affinity chromatography purification. After the antigen was emulsified with complete Freund's adjuvant, the stability of PCV2 subunit vaccine was studied as a comparison vaccine Va.
[0121] Patent application CN103122352A discloses a porcine circovirus type 2 recombinant baculovirus and a preparation method thereof. The method uses a baculovirus ex...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com