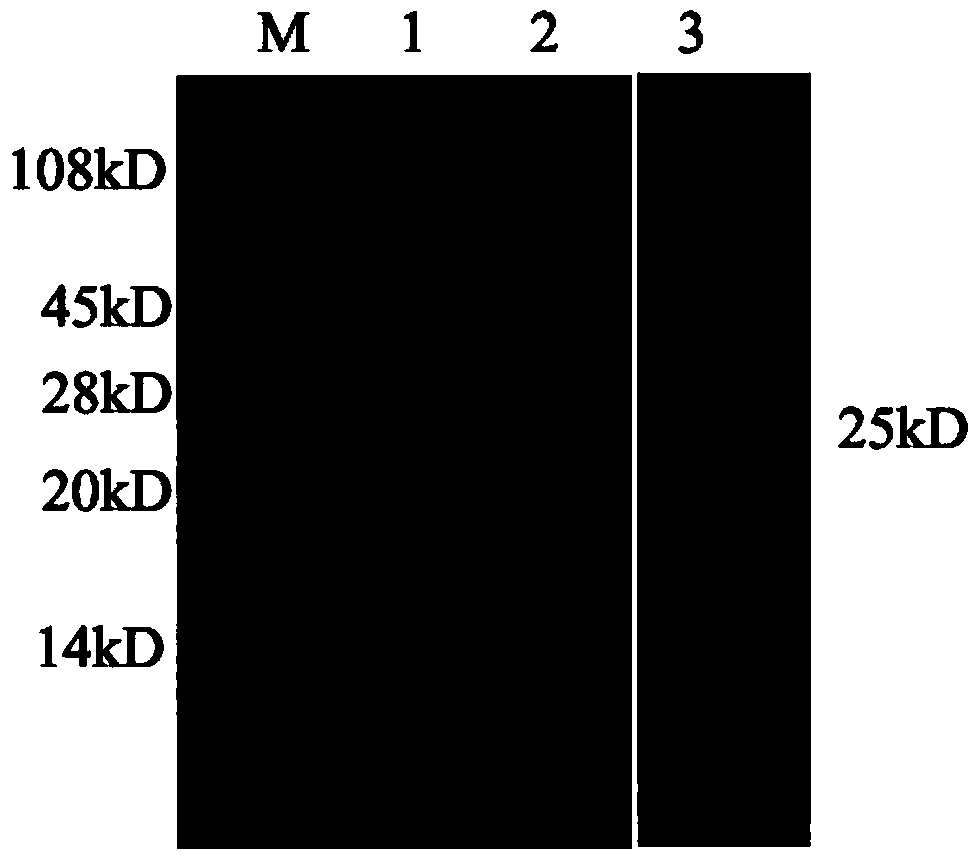
Hybridoma cell line and anti-canine distemper virus N protein monoclonal antibody produced through hybridoma cell line
A hybridoma cell line, monoclonal antibody technology, applied in the field of microorganism-related genetic engineering, can solve the problems of seldom use, unsafe allergy, danger of puppies and wild carnivores
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
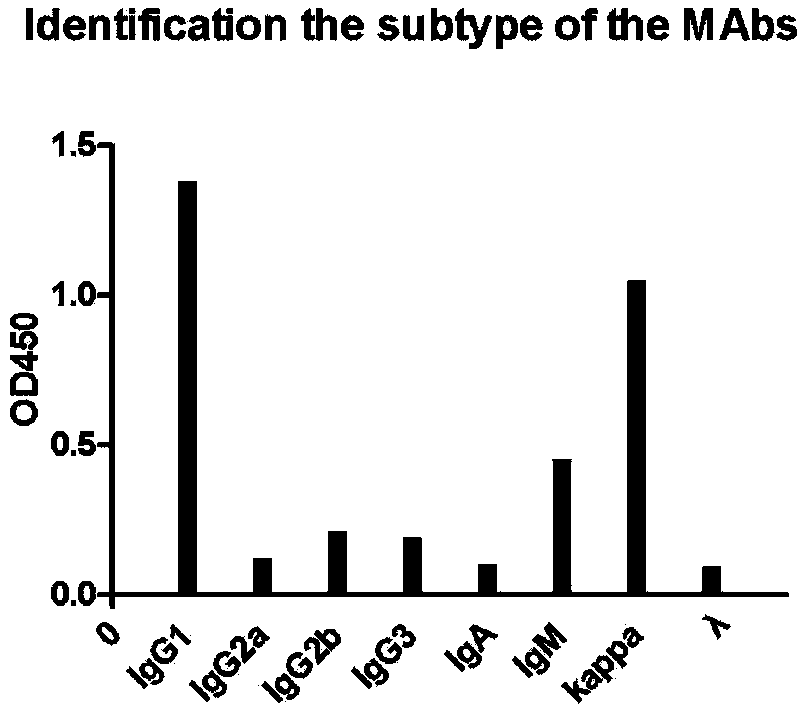
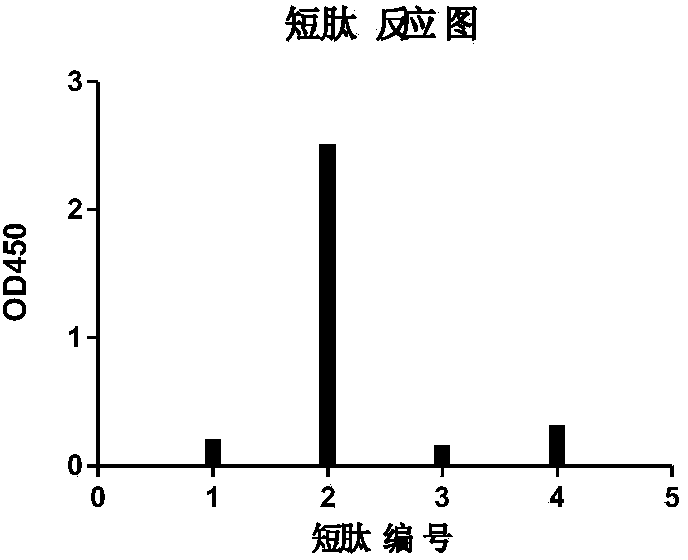
Image
Examples
specific Embodiment approach
[0022] Main experimental materials and sources
[0023] 1. Proteins, cells, viruses
[0024] The prokaryotic expressed and purified CDV-N protein, SP2 / 0 cells, Vero cells and CDV strains are all preserved by our laboratory.
[0025] 2. Main reagents and medicines
[0026] Fetal bovine serum and DMEM medium were purchased from GIBCO; diaminobenzidine (DAB) chromogenic kit, horseradish peroxidase (HRP)-labeled goat anti-mouse IgG antibody, FITC-labeled goat anti-mouse IgG antibody, glue The recovery kit was purchased from Kangwei Century Biotechnology Co., Ltd.; 50% PEG, 50×HAT, and 50×HT were purchased from Sigma; o-phenylenediamine and prestained protein marker were purchased from Fermentas; SBAClonotypingTM System / HRP antibody subclass Identification kits were purchased from Southern Biotechnology Company; plasmid extraction kits were purchased from AXYGEN Company, T-E1, reverse transcriptase, Ex Taq DNA polymerase, T4 DNA ligase, and HIS tag purification reagents were purc...
Embodiment 1
[0029] Prokaryotic expression and purification of embodiment 1 CDV-N protein
[0030] 1. Primer Design
[0031] According to the CDV-N gene sequence registered in Genbank (accession number: EF375619), design PCR amplification primers, the sequence is as follows: upstream primer 5-GAAACTATGTATCCGGCT-3, downstream primer 5-TGACTCACTCCATTCGGA-3
[0032] 2. Extraction and reverse transcription of CDV viral RNA
[0033]Viral genomic RNA was extracted from CDV-infected Vero cells by Trizol method as a template, and viral cDNA was synthesized by reverse transcription with random primers.
[0034] RNA extraction step by Trizol method: harvest Vero cells infected with CDV 1-5×10 7 Add 1mL Trizol, mix well, let stand at room temperature for 5min, add 0.2mL chloroform, shake vigorously for 15s, incubate at room temperature for 2-3min, centrifuge at 12000g, 4°C for 15min, carefully remove the upper layer of colorless liquid, add an equal volume of pre-cooled iso Propanol, after mixing,...
Embodiment 2
[0063] The preparation of embodiment 2 monoclonal antibody
[0064] 1. Mice Immunization
[0065] Five 6-week-old female BALB / c mice were immunized with affinity-purified prokaryotic recombinant CDV-N protein for 3 times with a two-week interval between each immunization. The immunization dose was 50 μg / mouse, and the immunization route was intraperitoneal immunization .
[0066] One week after the second and third immunizations, blood was collected from the tail of the mice, the serum was separated (4°C, 10,000 rpm, 20 min), and the antibody level was detected by indirect ELISA. Three days before cell fusion, BALB / c mice with good immune effect were boosted again, and each mouse was intraperitoneally injected with 50 μg of immune antigen.
[0067] 2. Cell Fusion
[0068] The feeder layer cells were prepared 1 day before fusion, and BALB / c mouse peritoneal macrophages were plated in 96-well cell culture plates according to conventional methods for use. Kill the mice to be ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com