Canine parvovirus trivalence subunit vaccine
A technology of canine parvovirus and subunit vaccines, which is applied in the direction of vaccines, viruses, antiviral agents, etc., can solve the problems of poor cross-protection, strong virulence, and inability to completely prevent canine parvovirus, so as to solve simultaneous infection and improve Effect of Antibody Titer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Example 1: Amplification and sequence analysis of VP2 gene
[0017] The suspected canine parvovirus materials collected in 2018 from 3 different regions were processed. In order to isolate the pathogen, the intestinal contents of the affected dogs were aseptically collected, centrifuged to collect the cleared bacteria, and then inoculated with cat kidney cells F81. After culturing for 5 days, the virus liquid was harvested. After the harvested virus liquid was purified, the analysis and detection of virus characteristics in terms of virus content, immunogenicity, specificity and purity were carried out. The results showed that the isolated virus strain had a specific reaction with canine parvovirus, and there was no bacteria, mycoplasma and Foreign virus contamination. The isolated 3 strains of virus were tested for HA with porcine erythrocytes, and the results were 9log2, 8log2, and 9log2 respectively. The HI test was carried out with the existing serum, and the cro...
Embodiment 2
[0026] Embodiment 2, the construction of the bacmid expressing VP2 gene
[0027] The synthesized genes of SEQ ID NO: 10, SEQ ID NO: 11, and SEQ ID NO: 12 were digested and connected into expression vectors. And the identified positive plasmids were transferred into DH10bac to construct the recombinant bacmids of CPV-2a, CPV-2b and CPV-2c.
[0028] 2.1 Cutting and optimization of VP2 gene
[0029] 2.2 Enzyme digestion reaction
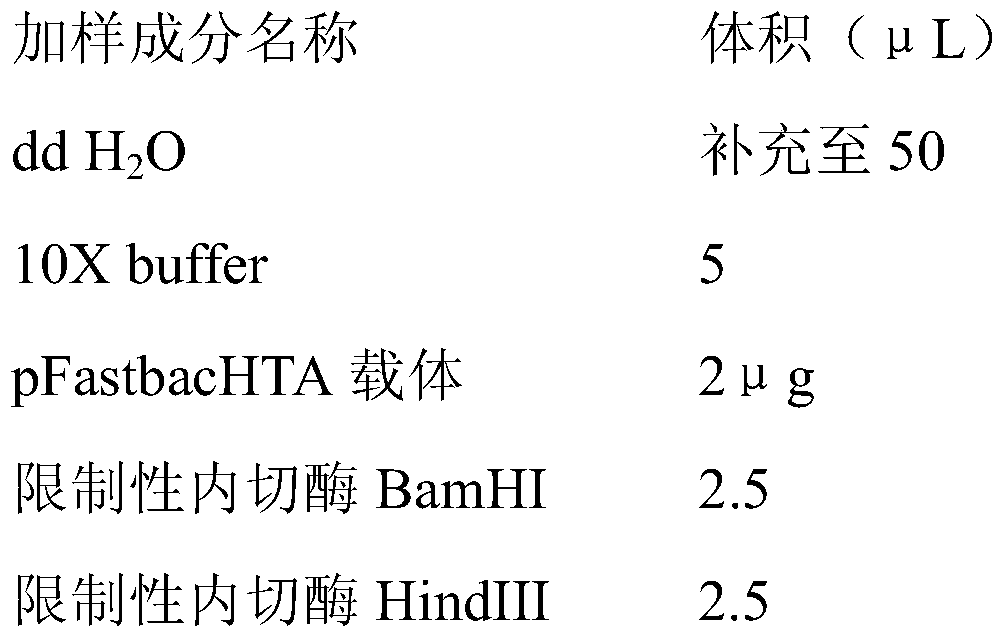
[0030] 2.2.1 Mark the 1.5mL EP tube to be used, and add and mix the sample in the 1.5mL EP tube according to the following table: the reaction system is 50 μL, and the sample addition is shown in the table below:
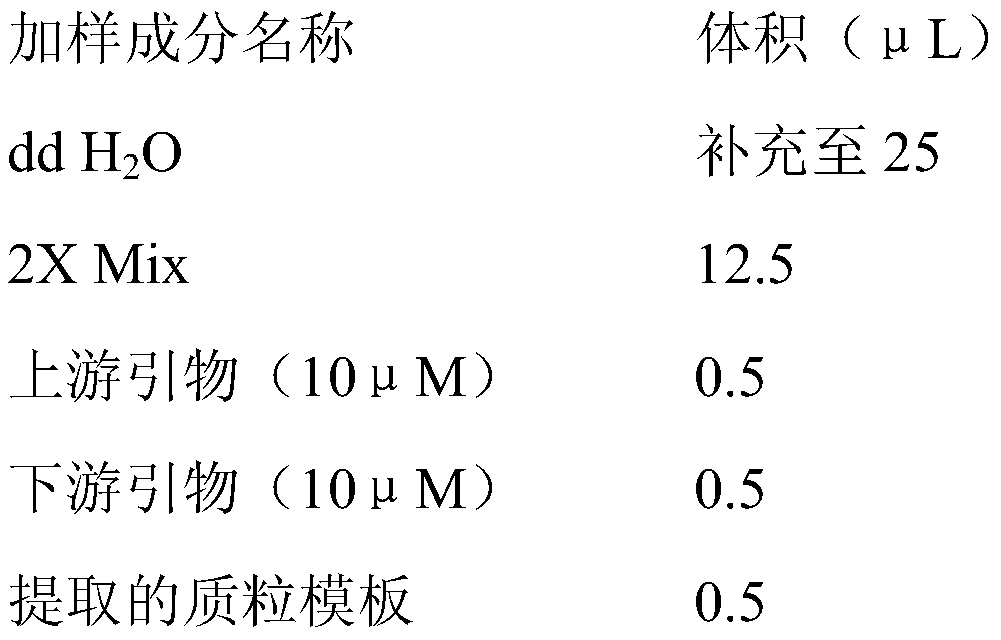
[0031]
[0032] 2.2.2 Place the 1.5mL EP tube in step 2.2.1 in a constant temperature water bath at 37°C for 2-3 hours.
[0033] 2.2.3 Gel recovery of double enzyme digestion products
[0034] Take out the above-mentioned double digestion system and perform agarose gel electrophoresis to recover the DNA fragments therein.
[0035] (1)...
Embodiment 3
[0097] Embodiment 3: SF9 cell transfection
[0098] (1) Preparation: UV sterilization in a biosafety cabinet for 30 minutes; TNM-FH culture solution was placed in a 27°C water bath and preheated to 27°C.
[0099] (2) Add 2 μg of the recombinant baculovirus prepared in Example 2 to 100 μl of TNM-FH culture medium without serum and double antibody, and mix well. Add 9 μl Cellfectin Reagent to 100 μl TNM-FH medium without serum and double antibody, and mix well. The liposomes were mixed with the recombinant DNA and allowed to stand at room temperature for 40 min.
[0100] (3) Take out the 6-well plate cells from the incubator at 27°C, discard the supernatant medium, wash the cells three times with pre-warmed TNM-FH culture medium, and discard the TNM-FH culture medium.
[0101] (4) Add 2 ml of 10% fetal bovine serum TNM-FH culture solution to each cell well.
[0102] (5) Gently add the mixture of recombinant DNA and liposomes into each well of cells, mix gently, and culture st...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com