Preparation method of chimeric vaccine by using Ii-Key active tetrapeptide carrying Fabricius bursa VP2 and newcastle disease HN antigen peptide epitope
A chimeric vaccine and bursa of fabric technology, applied in chemical instruments and methods, viral antigen components, peptides, etc., to achieve good immune effect, easy preparation, and strong specificity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
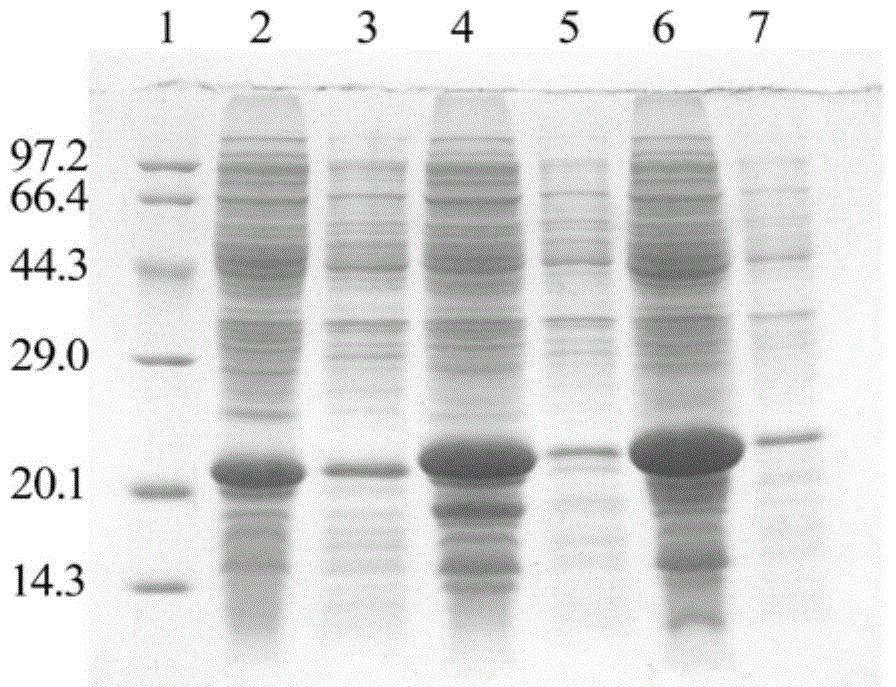
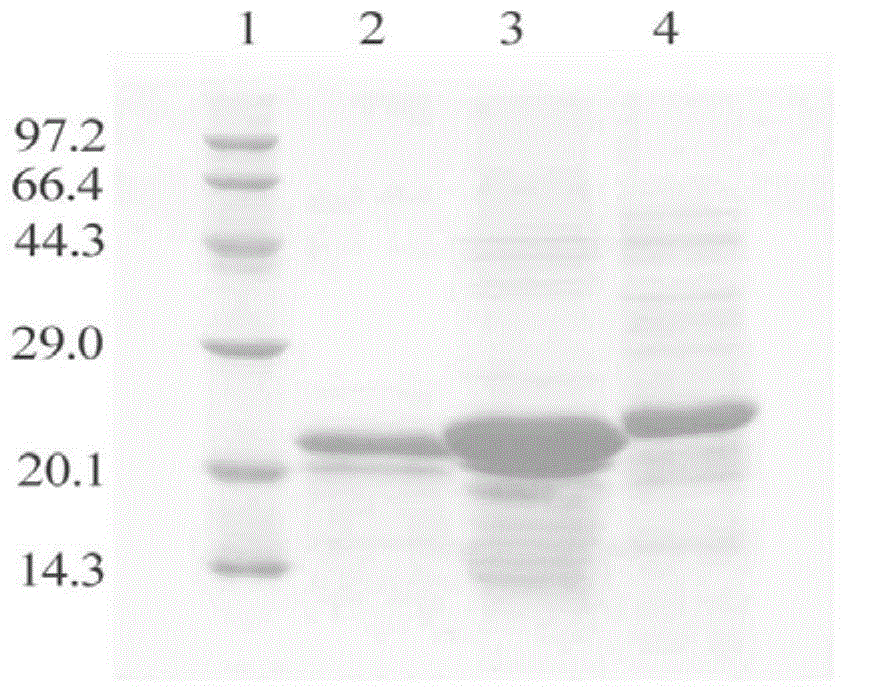
Image
Examples
Embodiment Construction
[0045] Below by embodiment the present invention will be further described, but do not limit the present invention.
[0046] The experimental methods in the following examples are conventional methods unless otherwise specified.
[0047] The percentages in the following examples are all mass percentages unless otherwise specified.
[0048] The proportions in the following examples are volume proportions unless otherwise specified.
[0049] 1. Chimera design and gene synthesis
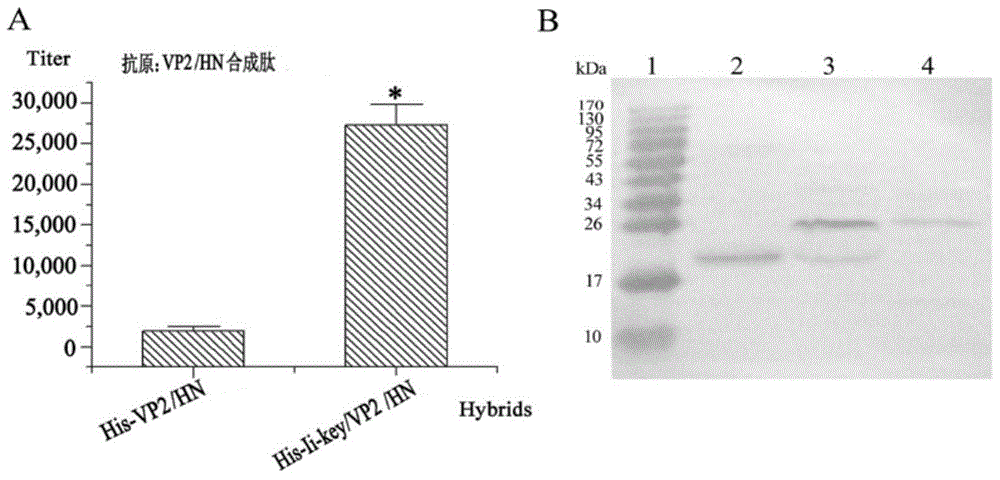
[0050] Design VP2 first 197-209 and HN 345-353 The tandem epitope, the epitope is linked by AAY linking peptide (Ala-Ala-Tyr), and the Ii-Key is then linked with the above tandem epitope. The specific sequence is as follows: 5'-CG gaattc CTTCGCATGAAGGCCGCCTACTGTGACAGCAGTGACAGGCCCAGAGTCTACACTATAACTGCCGCCTACGATGGACAAGATTACCAAATTCGGTGA gtcgac gcGT-3'
[0051] EcoR I was added at the 5' end of the gene sequence, a stop codon TGA and a SalI restriction site were added at the 3' end, the whole gene w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com